Periodic table Chapter 6 Order The periodic table

Periodic table Chapter 6
Order The periodic table is arranged in order of increasing atomic number § Identifies the element § Gives the number of protons § Gives the number of electrons in a neutral atom

Properties The properties within a period change as you move from left to right Elements within a group have similar physical and chemical properities § Example the Alkali Metals: § Highly reactive in water § Each element has one electron in its valence shell § Soft metals with low melting and low boiling points § Produce colored flames when burned
Metals nonmetals and metalloids Across a period the properties of elements become less metallic and more non metallic Metals- good conductors of heat and electric current 80% of the elements are metal
Metalloids- stair step line § Properties similar to those of metals and non metals
Non metals- upper right corner of periodic table (exception on Hydrogen) § Gases at room temperature § Poor conductors § Brittle
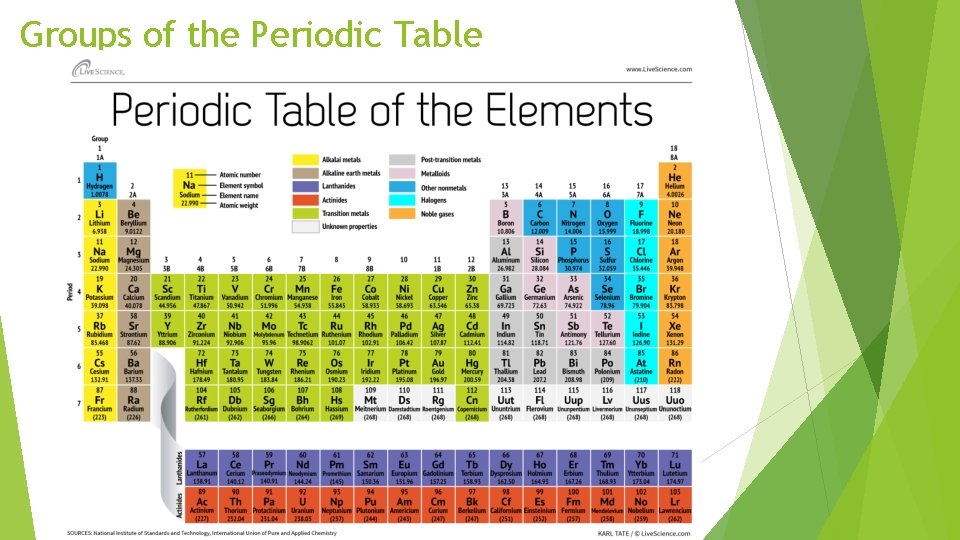
Groups of the Periodic Table
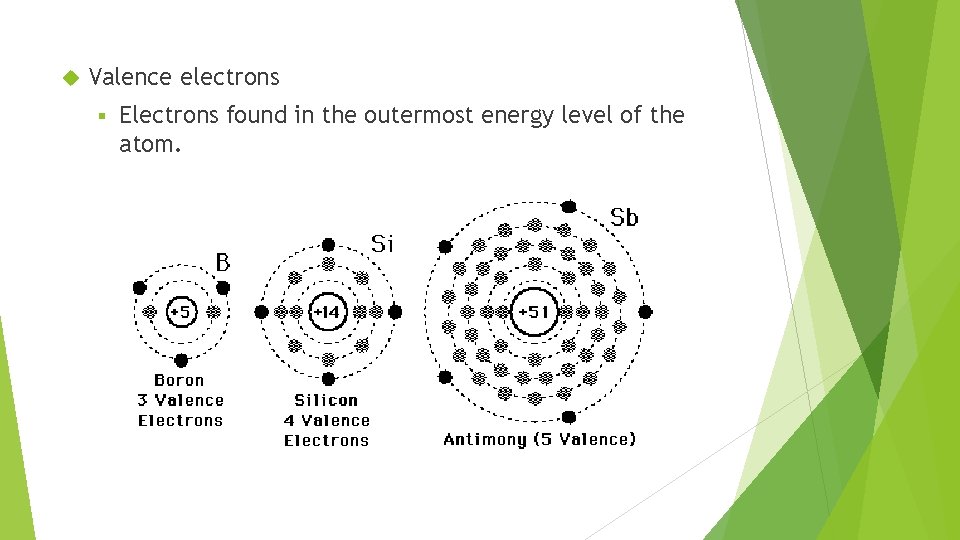
Valence electrons § Electrons found in the outermost energy level of the atom.
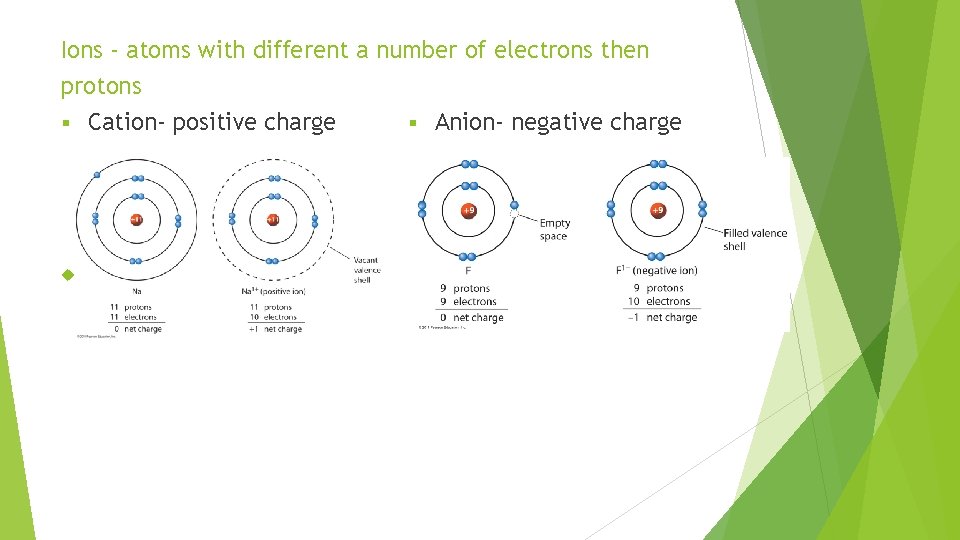
Ions - atoms with different a number of electrons then protons § Cation- positive charge § Anion- negative charge
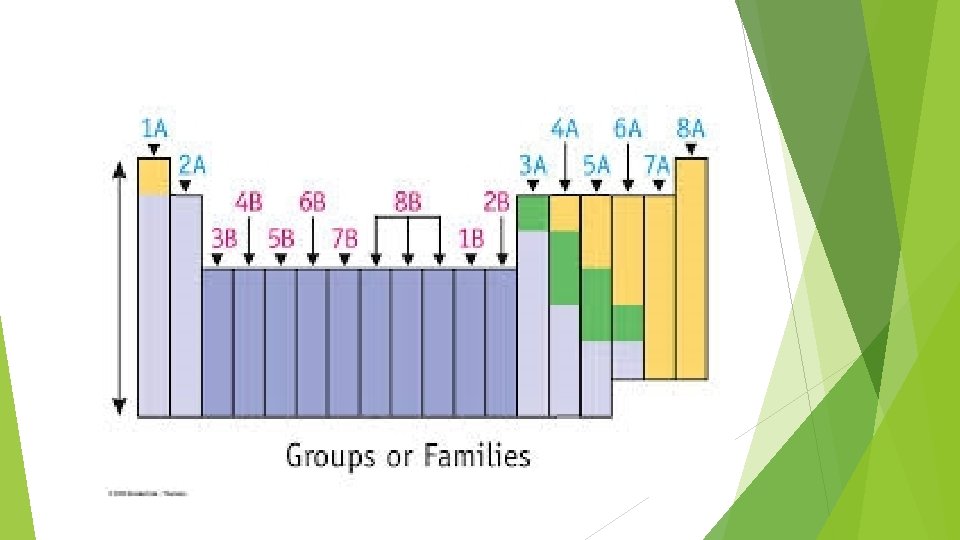

Reading the periodic table Alkali metals- 1 A Also tells you it has 1 electron in its valence shell When they are an ion they are a cation with a +1 charge (it wants to give one electron away) Alkali earth metals – 2 A Also tells you it has 2 electrons in its valence shell When they are an ion they are a cation with a +2 charge Group – 3 A +3 charge

Reading the periodic table Halogens – 7 A Also tells you it has 7 electrons in its valence shell Salts when bonded to a metal When it’s a anion it has a -1 charge (it wants one electron) Nobel gases – 8 A Valence shell is full – VERY STABLE Transition metals In d orbital Ag+1 Zn+2
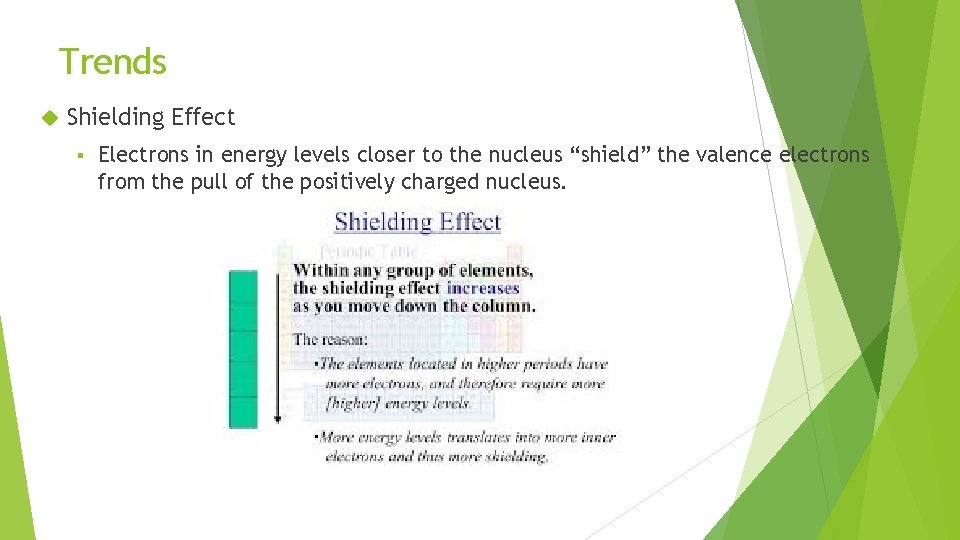
Trends Shielding Effect § Electrons in energy levels closer to the nucleus “shield” the valence electrons from the pull of the positively charged nucleus.
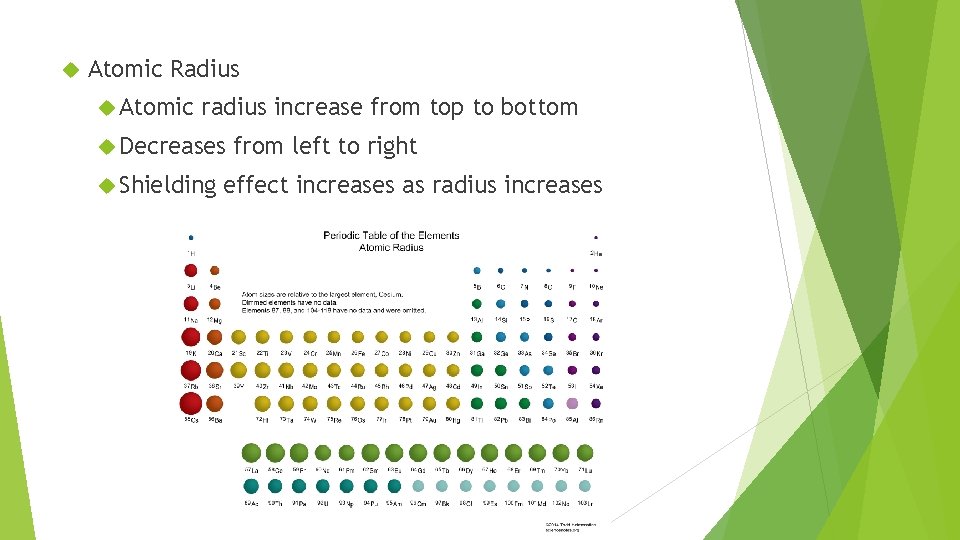
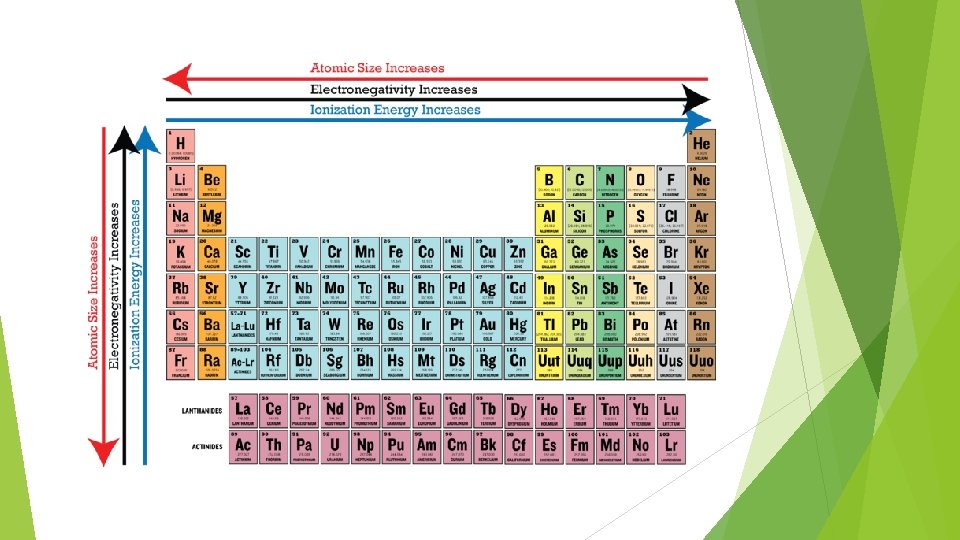
Atomic Radius Atomic radius increase from top to bottom Decreases Shielding from left to right effect increases as radius increases
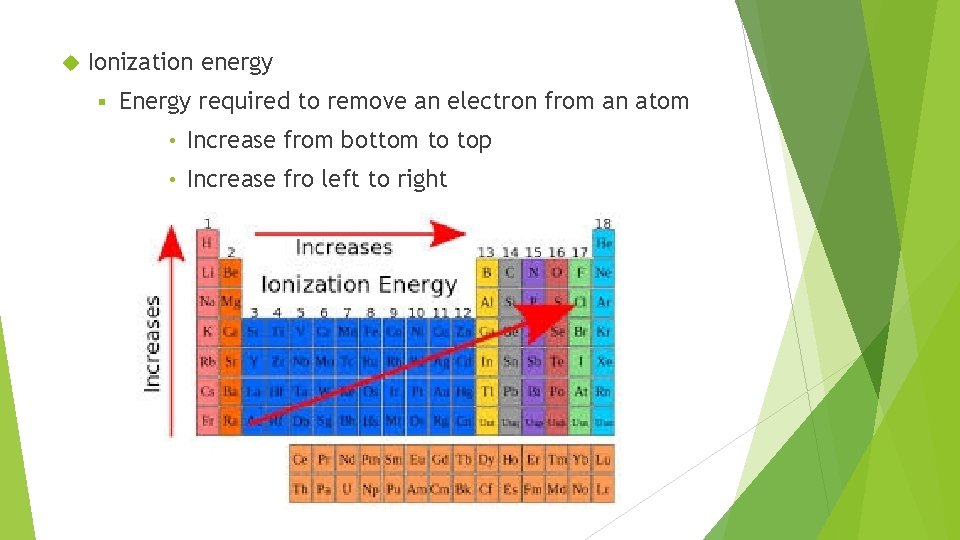
Ionization energy § Energy required to remove an electron from an atom • Increase from bottom to top • Increase fro left to right
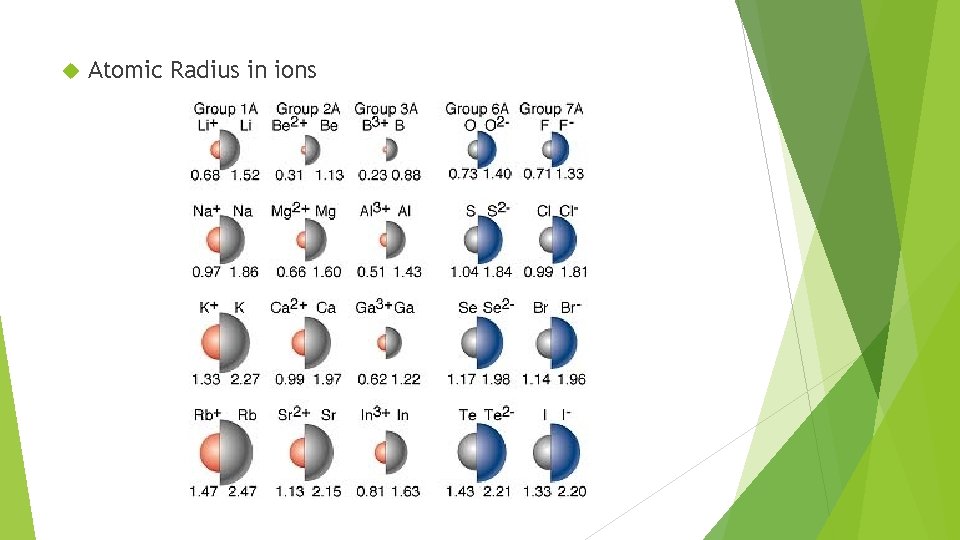
Atomic Radius in ions
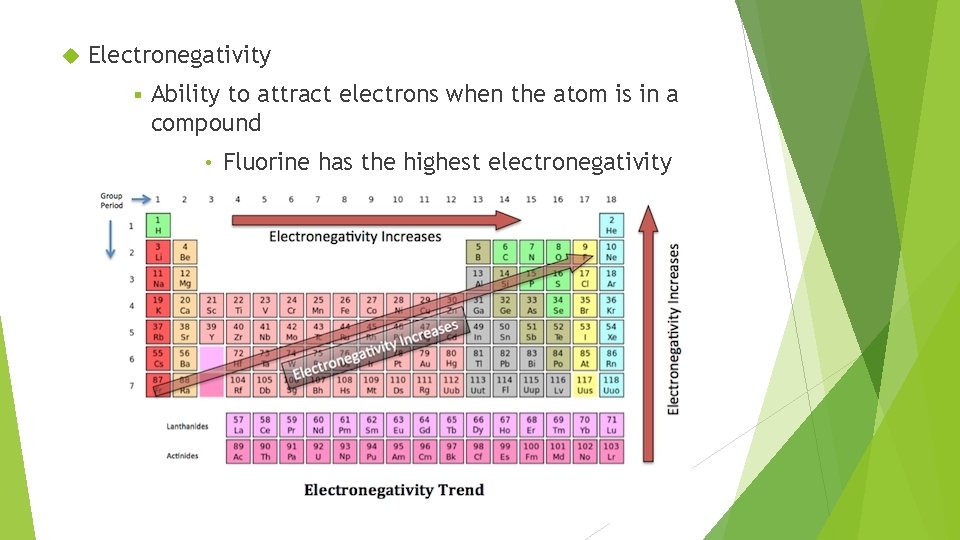
Electronegativity § Ability to attract electrons when the atom is in a compound • Fluorine has the highest electronegativity
- Slides: 18