Periodic Table Breakdown Dmitri Mendeleev Father of the
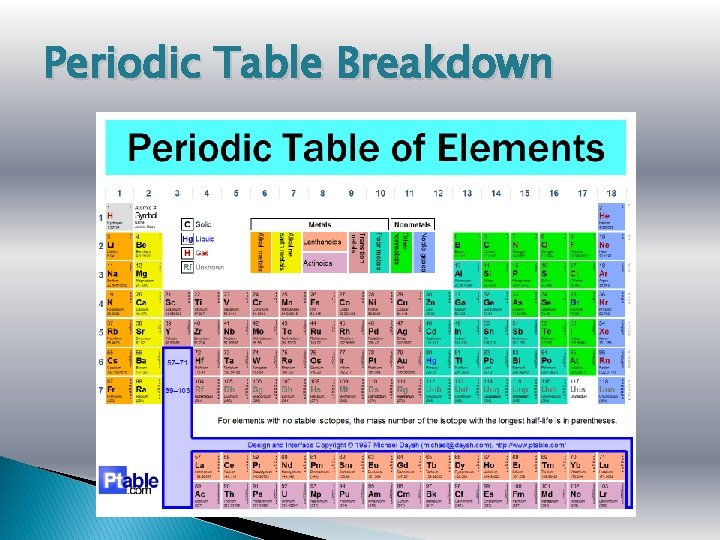
Periodic Table Breakdown

Dmitri Mendeleev � Father of the Periodic Table � Russian scientist circa mid 1800 s � Arranged Periodic Table by atomic weights & chemical properties � SONG!!!!
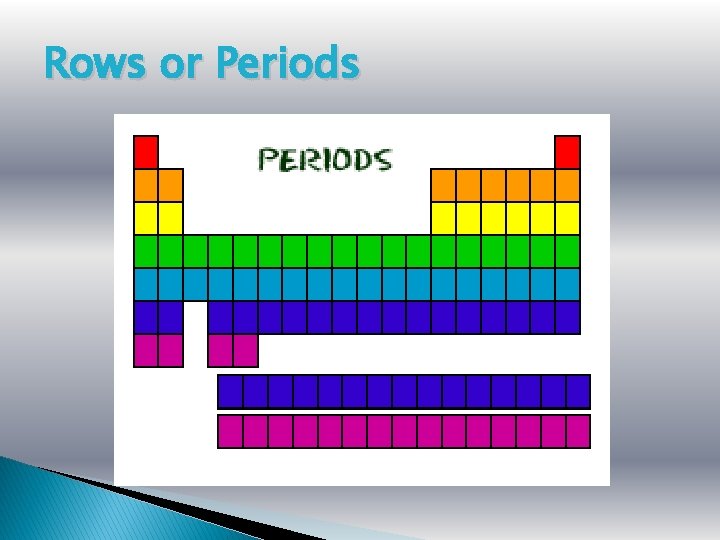
Rows or Periods

Groups or Families
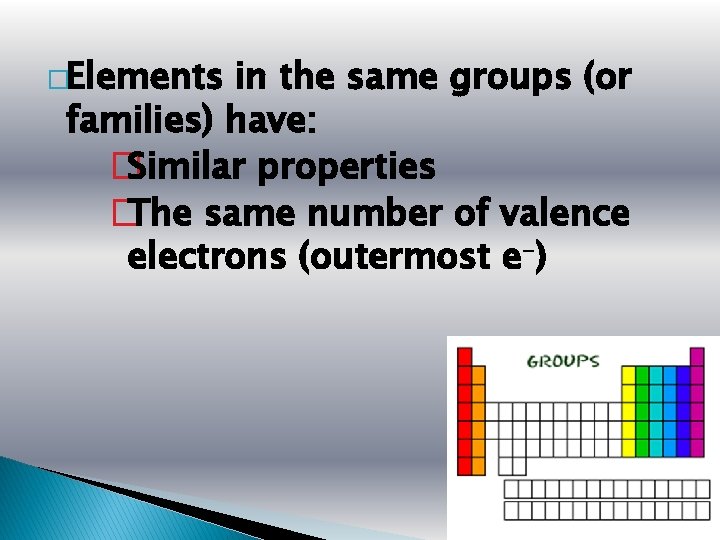
�Elements in the same groups (or families) have: � Similar properties � The same number of valence electrons (outermost e-)
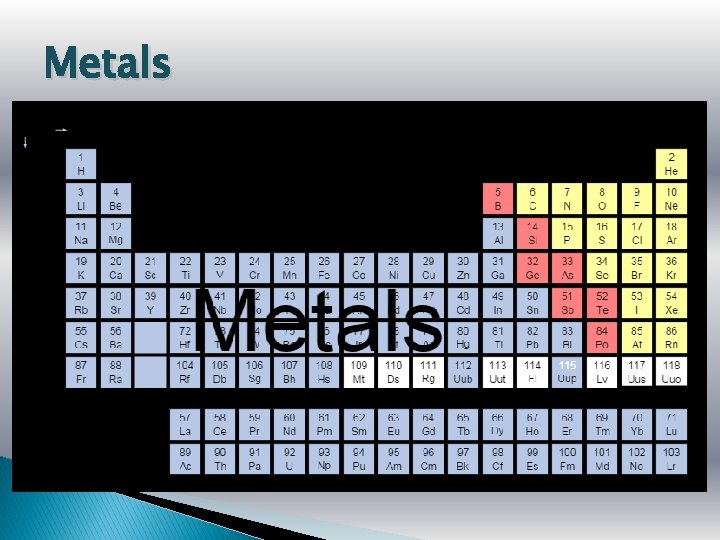
Metals

Metals � Metals are lustrous (shiny), malleable, ductile, and are good conductors of heat and electricity. � They are mostly solids at room temp. � What is one exception? Hydrogen – it doesn’t have a family
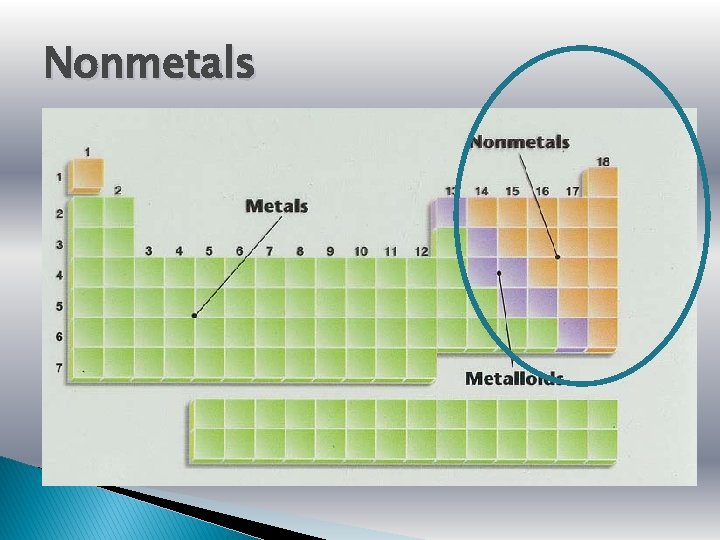
Nonmetals

Nonmetals � Nonmetals are the opposite. � They are dull, brittle, nonconductors (insulators). � Some are solid, but many are gases, and Bromine is a liquid.
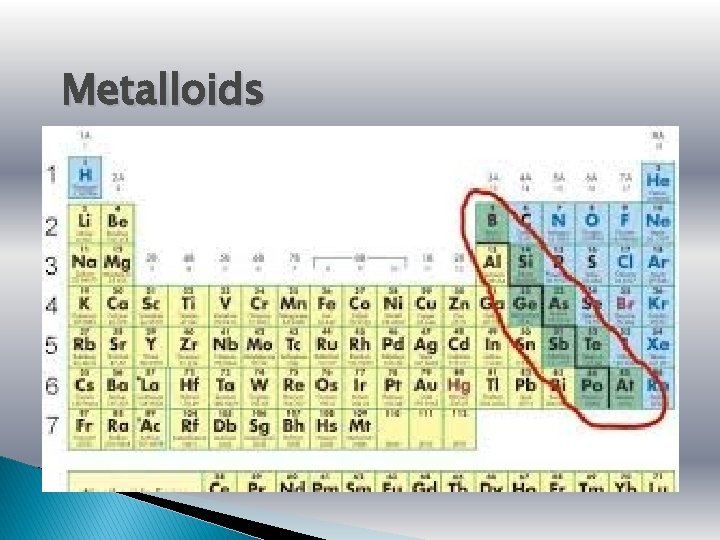
Metalloids

Metalloids � Metalloids, aka semi-metals are just that. � They have characteristics of both metals and nonmetals. � They are shiny but brittle. � And they are semiconductors (can conduct electricity under some conditions but not others, making it a good medium for the control of electrical current) Silicon – used in electronic devices and solar panels
� Can you sing THIS?
Periodic Table Groups/ Families
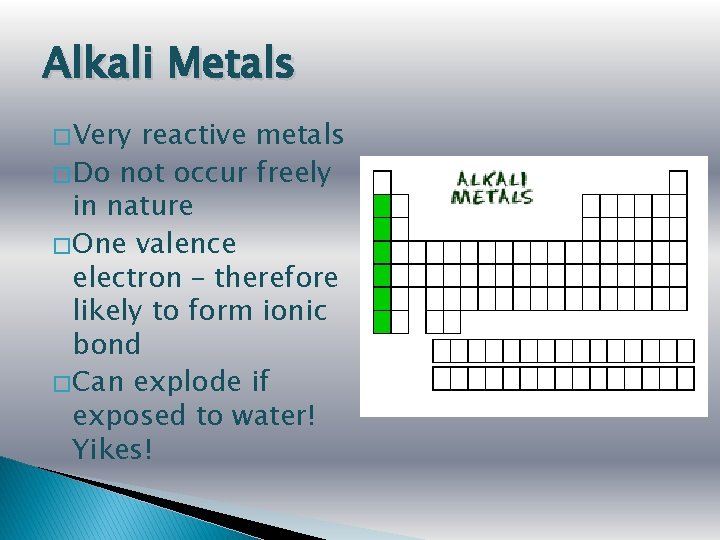
Alkali Metals � Very reactive metals � Do not occur freely in nature � One valence electron – therefore likely to form ionic bond � Can explode if exposed to water! Yikes!

“Chemistry is a bit like that: powerful enough to do great things in the world, but also dangerous enough to do terrible things just as easily. If you don’t respect it, chemistry bites. ” ~Theodore Gray~

Alkaline Earth Metals � Oxidation number of +2 making them very reactive, but tamer than Alkali metals � Because so reactive, alkaline earth metals are NOT found freely in nature
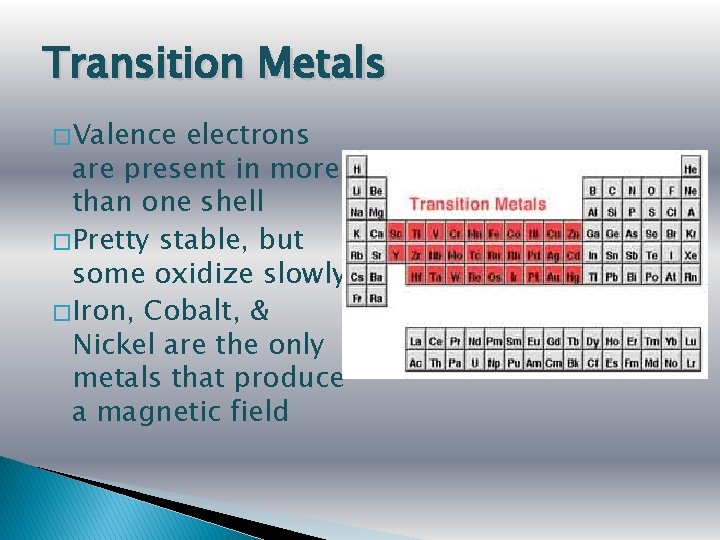
Transition Metals � Valence electrons are present in more than one shell � Pretty stable, but some oxidize slowly � Iron, Cobalt, & Nickel are the only metals that produce a magnetic field
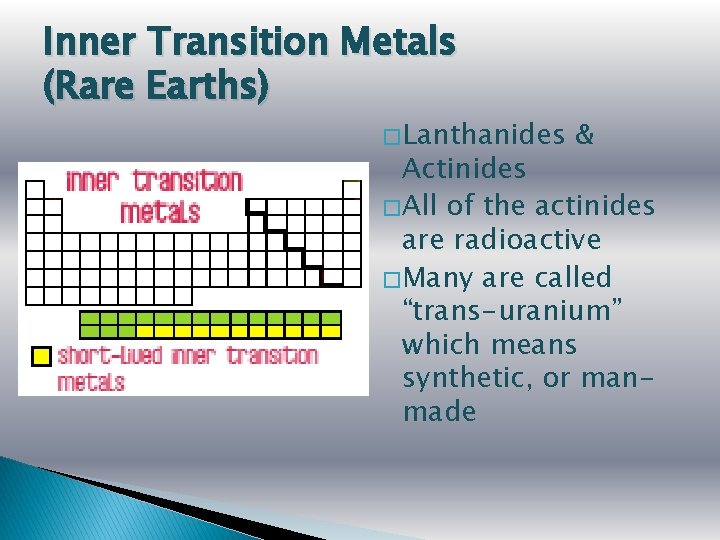
Inner Transition Metals (Rare Earths) � Lanthanides & Actinides � All of the actinides are radioactive � Many are called “trans-uranium” which means synthetic, or manmade
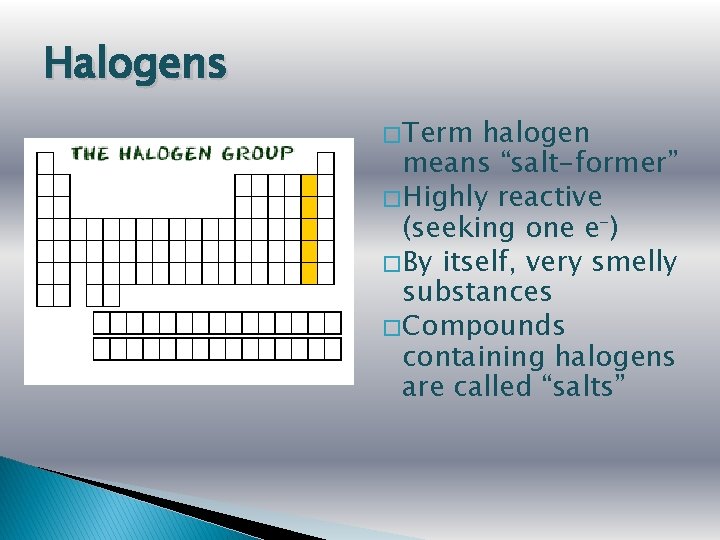
Halogens � Term halogen means “salt-former” � Highly reactive (seeking one e-) � By itself, very smelly substances � Compounds containing halogens are called “salts”
Noble Gases � Full outer shell – 8 valence electrons � Noble gases do not form compounds readily � Elements are stable on its own

Elements, Compounds, Mixtures A little review
Elements � Made of atoms of all the same type � 118 unique versions � 92 naturally occurring elements

Elements � Hydrogen is the most common element � Most reactive metal francium � Most reactive nonmetal fluorine
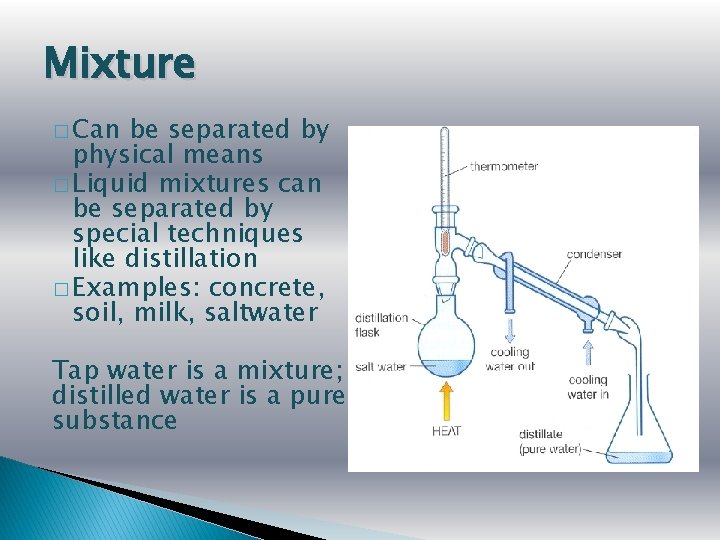
Mixture � Can be separated by physical means � Liquid mixtures can be separated by special techniques like distillation � Examples: concrete, soil, milk, saltwater Tap water is a mixture; distilled water is a pure substance
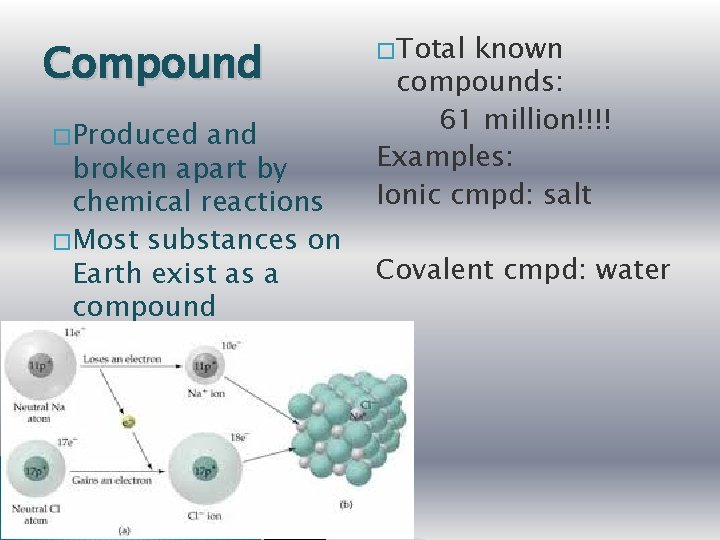
Compound � Produced and broken apart by chemical reactions � Most substances on Earth exist as a compound � Total known compounds: 61 million!!!! Examples: Ionic cmpd: salt Covalent cmpd: water

Homogeneous Mixture vs. Heterogeneous Mixture � Homogeneous � Heterogenous Has a uniform composition and properties throughout. Made of different substances that remain physically separate
- Slides: 26