PERIODIC TABLE BASICS This Photo by Unknown Author
PERIODIC TABLE BASICS This Photo by Unknown Author is licensed under CC BY-SA-NC
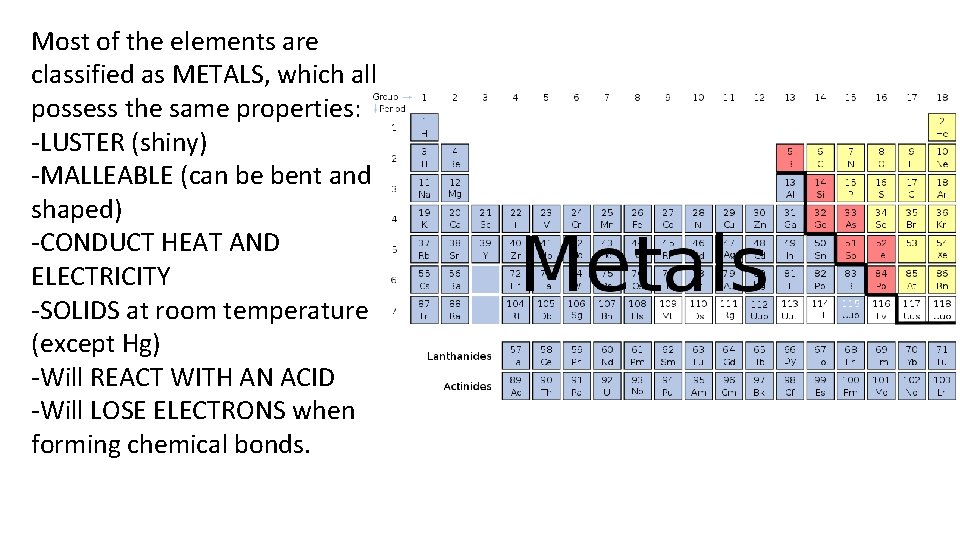
Most of the elements are classified as METALS, which all possess the same properties: -LUSTER (shiny) -MALLEABLE (can be bent and shaped) -CONDUCT HEAT AND ELECTRICITY -SOLIDS at room temperature (except Hg) -Will REACT WITH AN ACID -Will LOSE ELECTRONS when forming chemical bonds.
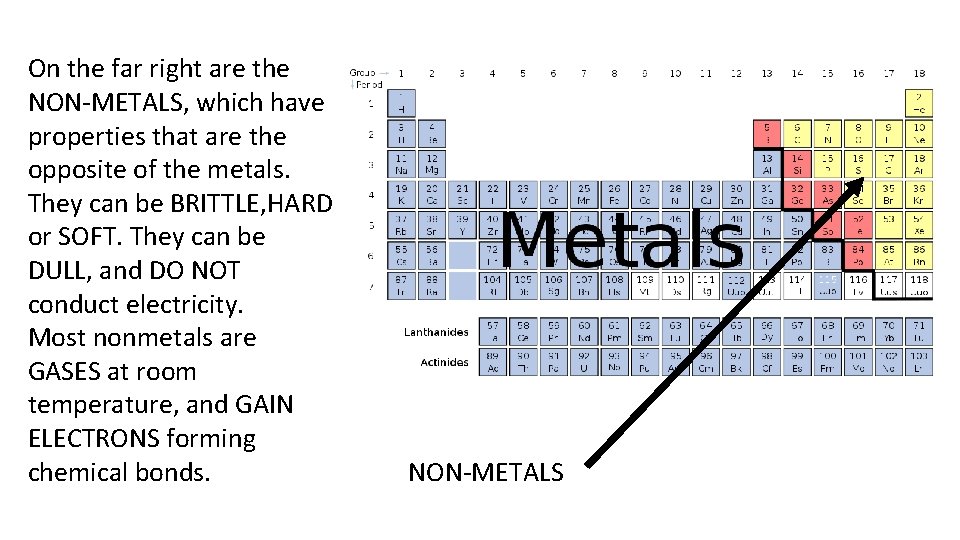
On the far right are the NON-METALS, which have properties that are the opposite of the metals. They can be BRITTLE, HARD or SOFT. They can be DULL, and DO NOT conduct electricity. Most nonmetals are GASES at room temperature, and GAIN ELECTRONS forming chemical bonds. NON-METALS
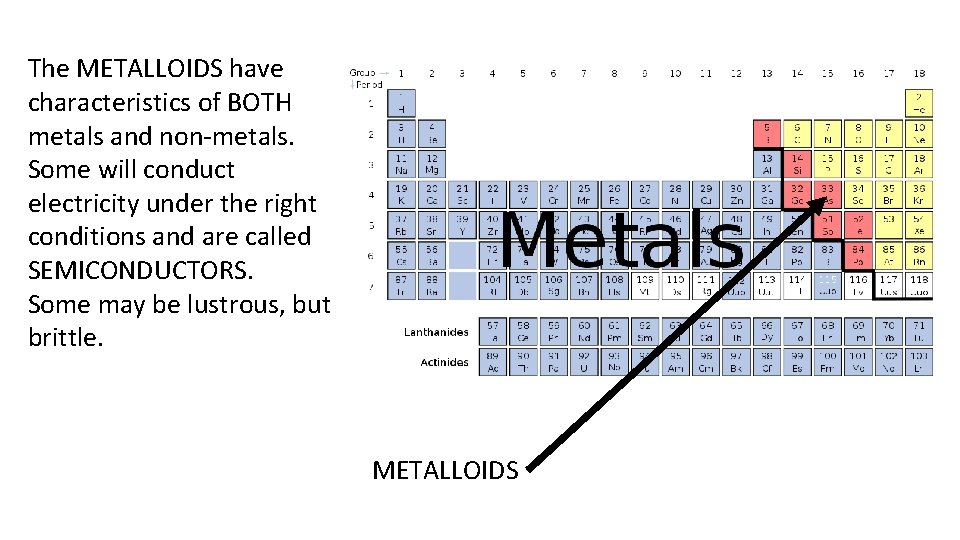
The METALLOIDS have characteristics of BOTH metals and non-metals. Some will conduct electricity under the right conditions and are called SEMICONDUCTORS. Some may be lustrous, but brittle. METALLOIDS
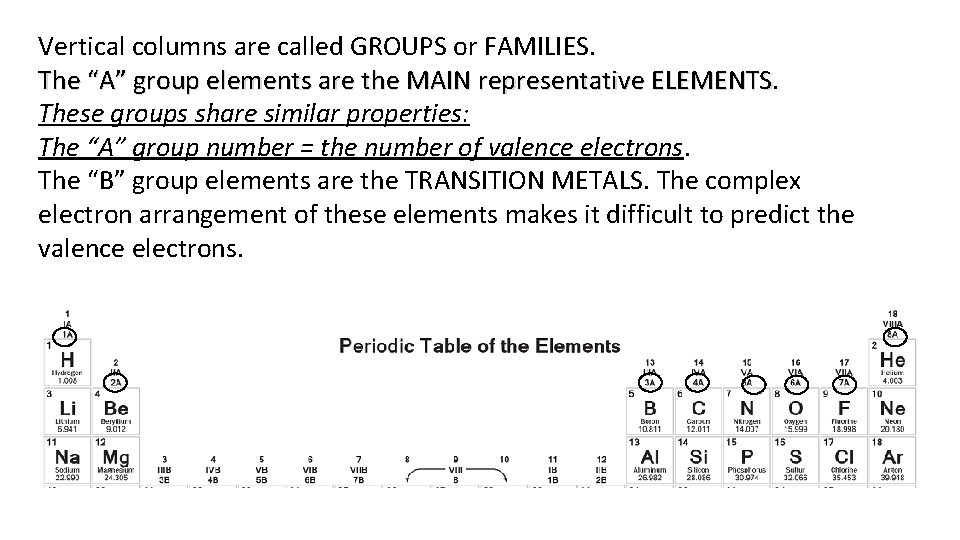
Vertical columns are called GROUPS or FAMILIES. The “A” group elements are the MAIN representative ELEMENTS These groups share similar properties: The “A” group number = the number of valence electrons. The “B” group elements are the TRANSITION METALS. The complex electron arrangement of these elements makes it difficult to predict the valence electrons.
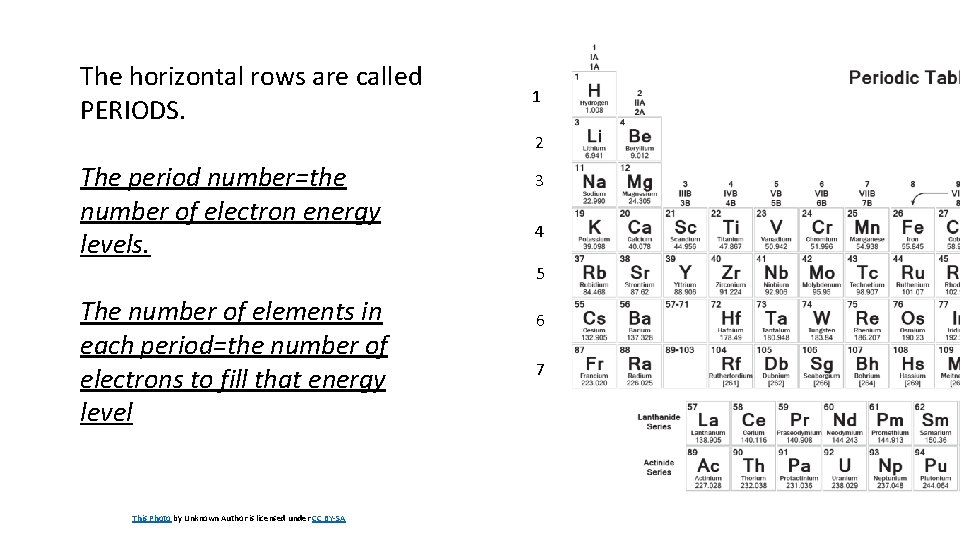
The horizontal rows are called PERIODS. 1 2 The period number=the number of electron energy levels. 3 4 5 The number of elements in each period=the number of electrons to fill that energy level This Photo by Unknown Author is licensed under CC BY-SA 6 7

Negatively charged ELECTRONS are attracted to the positively charged nucleus by an ELECTROMAGNETIC FORCE. Electrons have more energy the further they are from the nucleus. GROUND STATE the lowest energy state of an atom or other particle EXCITED STATE When an electron is in a higher energy level than it’s ground state
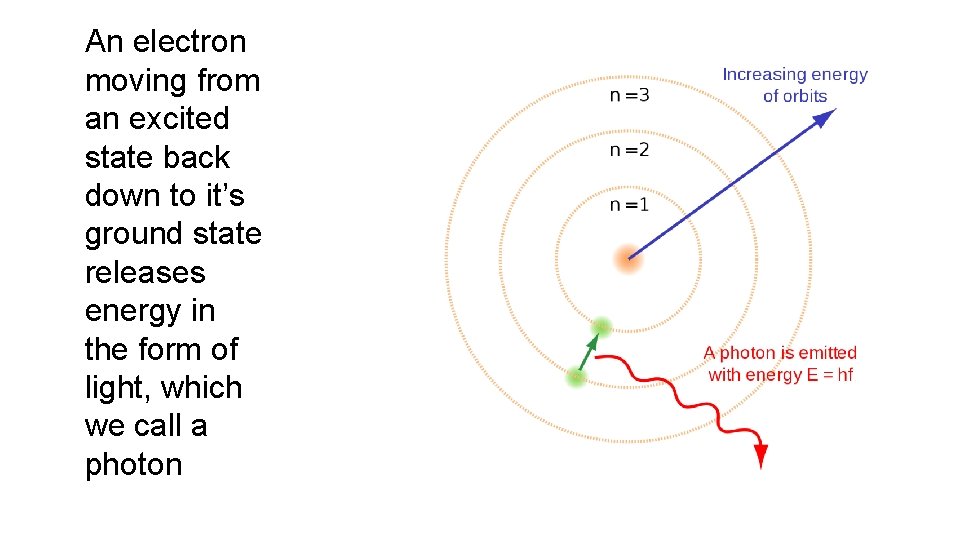
An electron moving from an excited state back down to it’s ground state releases energy in the form of light, which we call a photon

In basic chemistry, we learn : The first energy level can hold 2 electrons The second energy level can hold 8 electrons The third energy level can hold 18 electrons The fourth energy level can hold 32 electrons (2) (6) (10) (2) (14) (6) (10) (2) (6) (2)
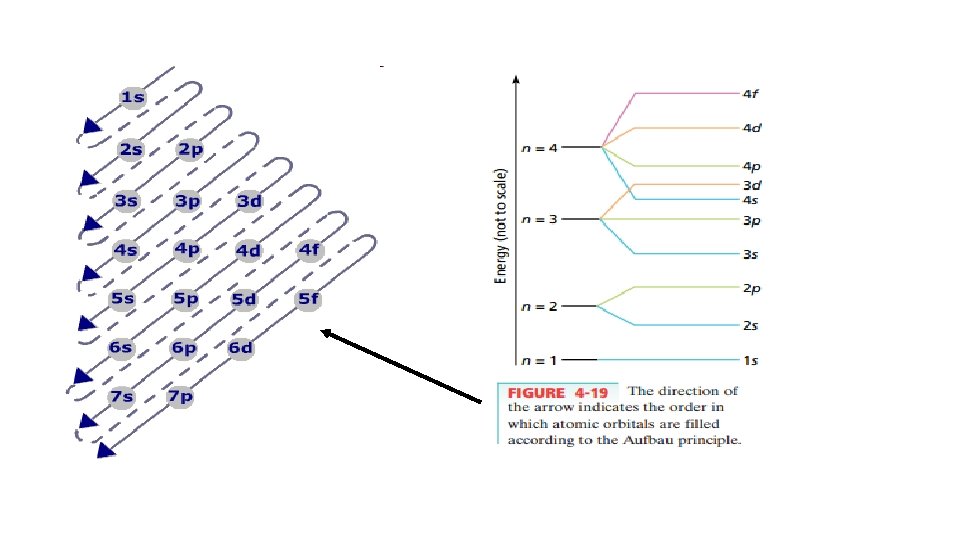
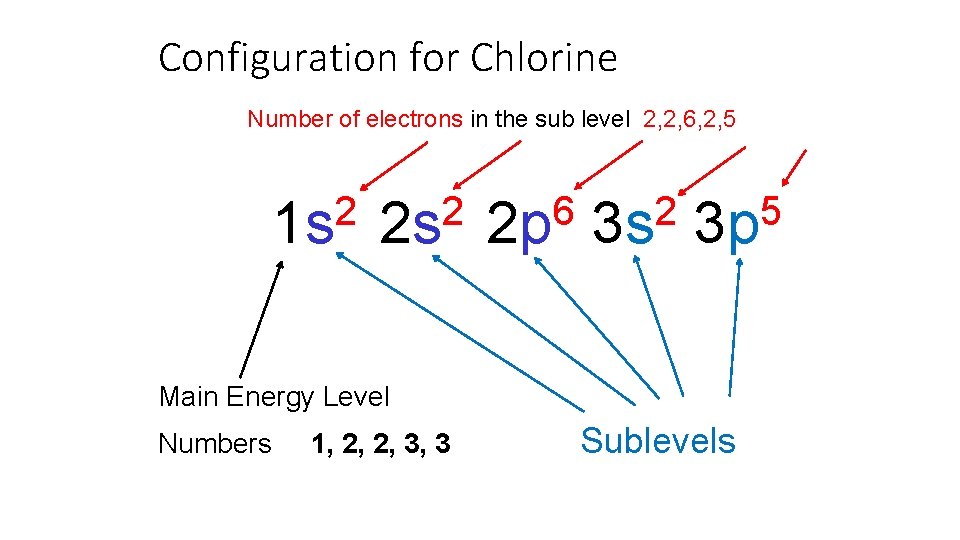
Configuration for Chlorine Number of electrons in the sub level 2, 2, 6, 2, 5 2 1 s 2 2 s 6 2 p 2 3 s 5 3 p Main Energy Level Numbers 1, 2, 2, 3, 3 Sublevels

Refer to your periodic table. Is Chlorine a metal or non metal? How many electron energy levels does Chlorine have? How many valence electrons does Chlorine have? This classification of element will gain electrons when bonding.
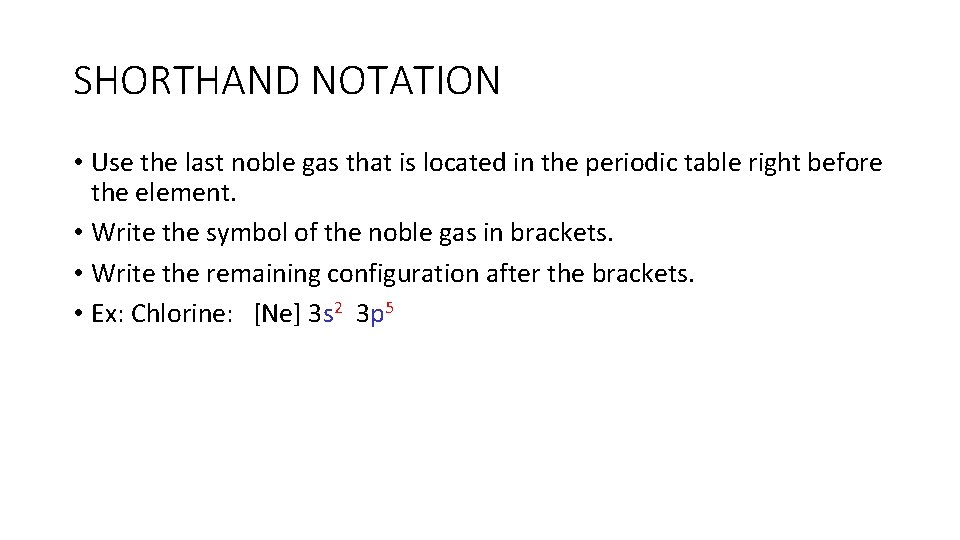
SHORTHAND NOTATION • Use the last noble gas that is located in the periodic table right before the element. • Write the symbol of the noble gas in brackets. • Write the remaining configuration after the brackets. • Ex: Chlorine: [Ne] 3 s 2 3 p 5
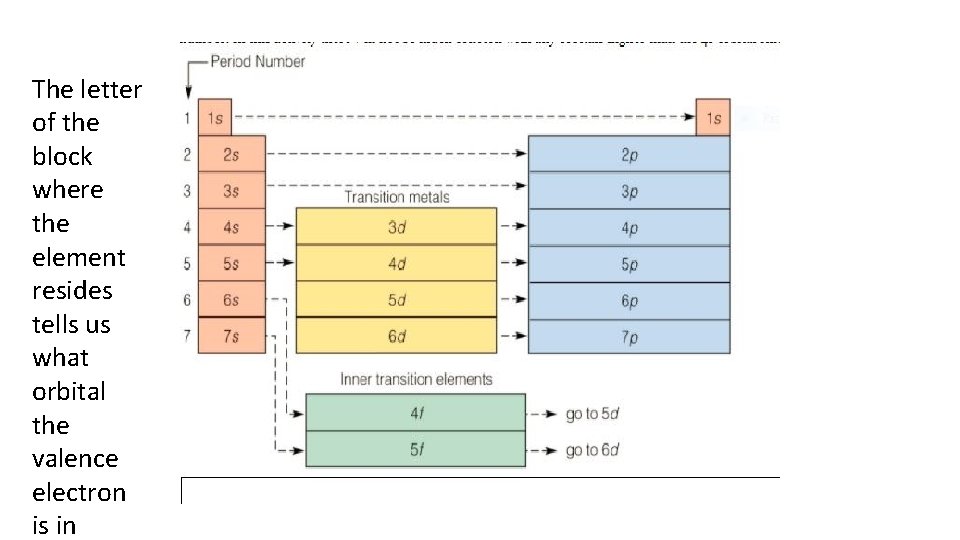
The letter of the block where the element resides tells us what orbital the valence electron is in
- Slides: 14