Periodic Table Basics Period Row on the periodic
Periodic Table Basics • Period – Row on the periodic table – Tells energy level • Principal Quantum Number Episode 402
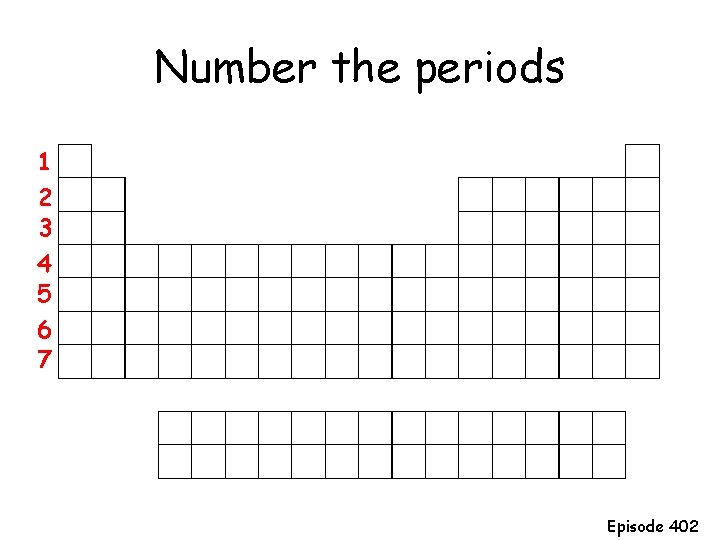
Number the periods 1 2 3 4 5 6 7 Episode 402
Periodic Table Basics • Period – Row on the periodic table – Tells energy level • Principal Quantum Number • Family or Group – Column on the periodic table – For columns 1 -2 and 13 -18, the number in the one’s place represents number of valence electrons Episode 402
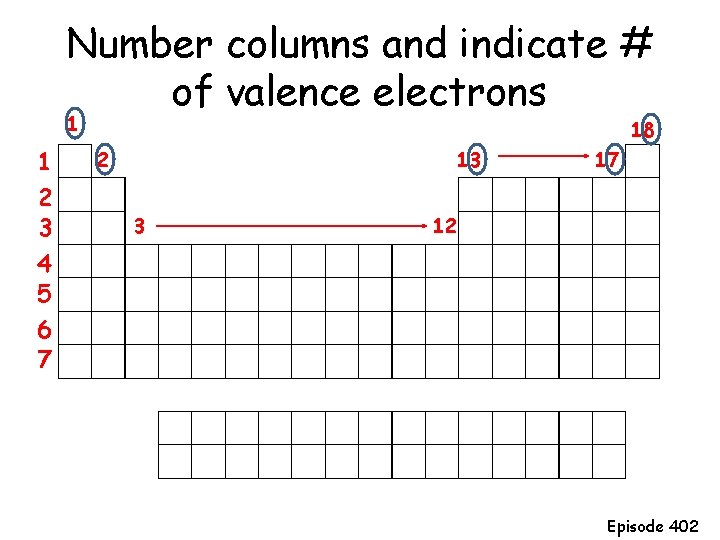
Number columns and indicate # of valence electrons 1 1 2 3 4 5 6 7 18 2 13 3 17 12 Episode 402
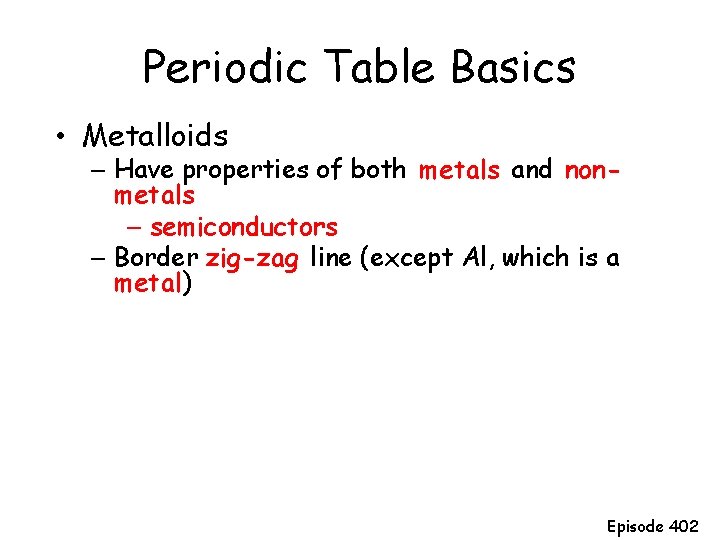
Periodic Table Basics • Metalloids – Have properties of both metals and nonmetals – semiconductors – Border zig-zag line (except Al, which is a metal) Episode 402

Mark the zig-zag border and label metalloids 1 1 2 3 4 5 6 7 18 2 13 B 3 12 metals 17 Non-metals Si Ge As Sb Te Po At Episode 402
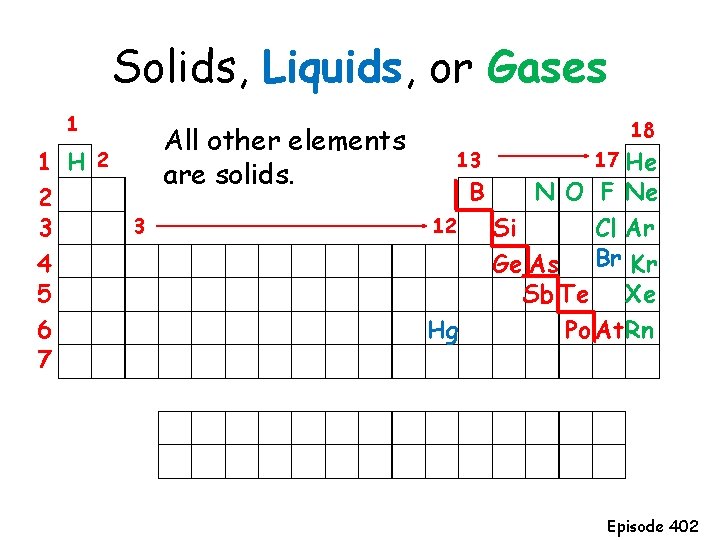
Solids, Liquids, or Gases 1 1 H 2 2 3 3 4 5 6 7 All other elements are solids. 18 13 B 12 Hg 17 He N O F Ne Si Cl Ar Br Kr Ge As Sb Te Xe Po At Rn Episode 402
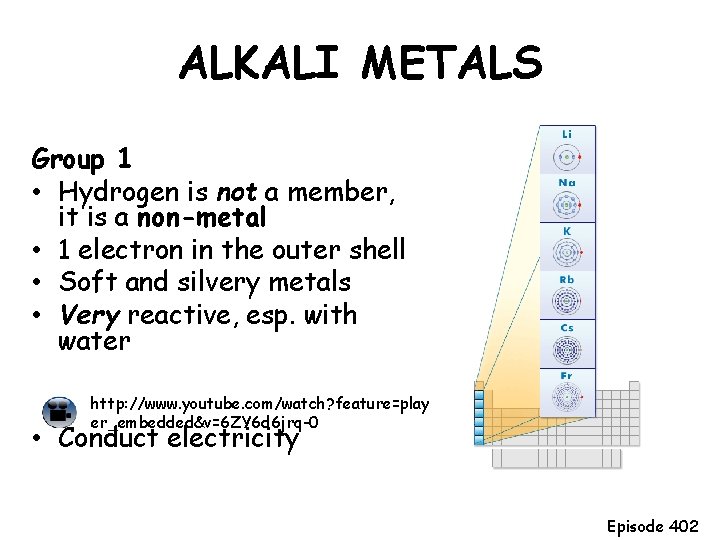
ALKALI METALS Group 1 • Hydrogen is not a member, it is a non-metal • 1 electron in the outer shell • Soft and silvery metals • Very reactive, esp. with water http: //www. youtube. com/watch? feature=play er_embedded&v=6 ZY 6 d 6 jrq-0 • Conduct electricity Episode 402

ALKALINE EARTH METALS Group 2 • 2 electrons in the outer shell • White and malleable • Reactive, but less than Alkali metals • Conduct electricity http: //www. youtube. com/watch? v=B 2 ZPrg 9 IVEo&fe ature=player_embedded Episode 402
TRANSITION METALS Groups 3 -12 �Good conductors of heat and electricity. �Some are used for jewelry. �The transition metals are able to put up to 32 electrons in their second to last shell. �Can bond with many elements to form a variety of compounds. Episode 402
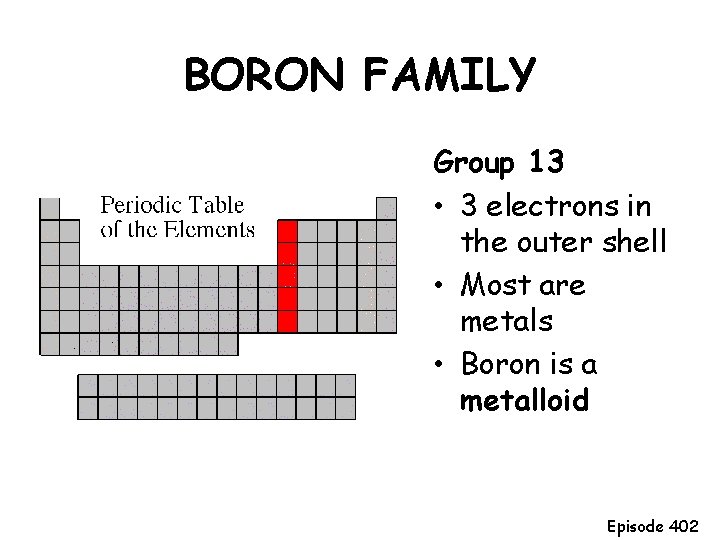
BORON FAMILY Group 13 • 3 electrons in the outer shell • Most are metals • Boron is a metalloid Episode 402
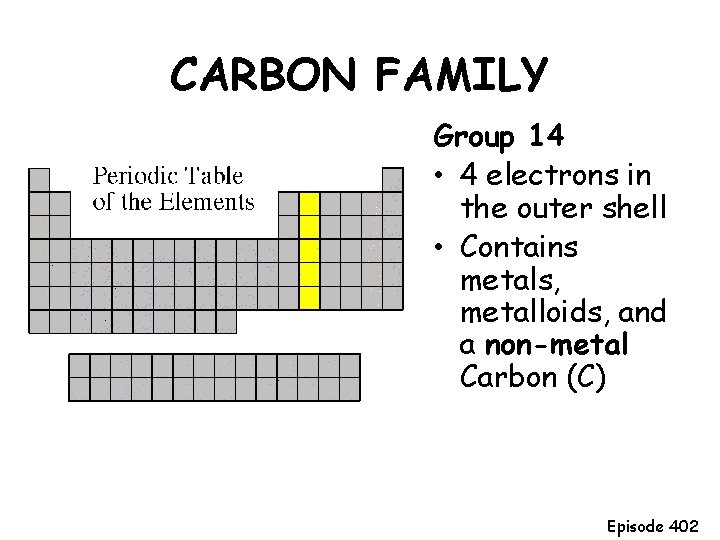
CARBON FAMILY Group 14 • 4 electrons in the outer shell • Contains metals, metalloids, and a non-metal Carbon (C) Episode 402
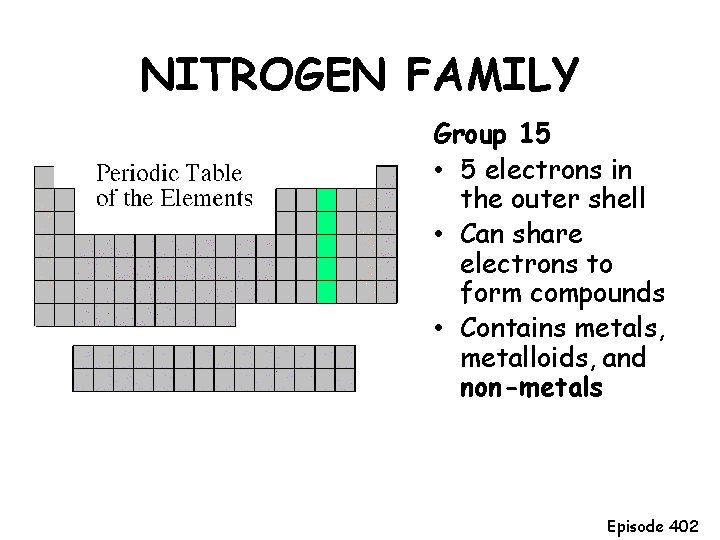
NITROGEN FAMILY Group 15 • 5 electrons in the outer shell • Can share electrons to form compounds • Contains metals, metalloids, and non-metals Episode 402
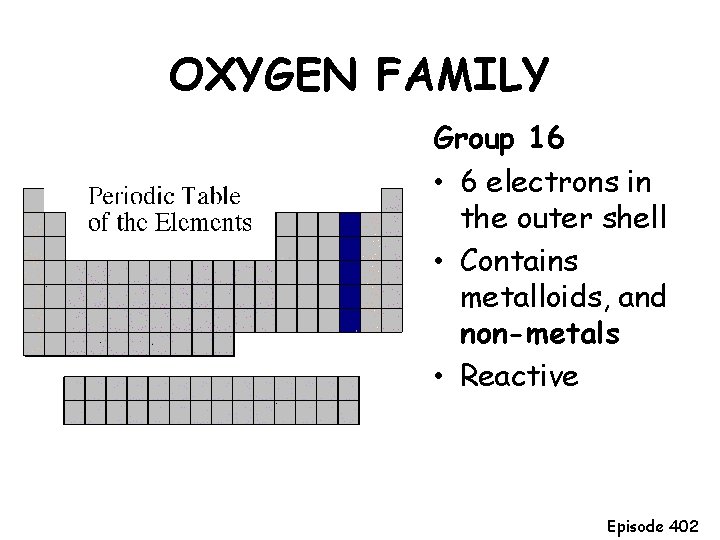
OXYGEN FAMILY Group 16 • 6 electrons in the outer shell • Contains metalloids, and non-metals • Reactive Episode 402
Halogens Group 17 • 7 electrons in the outer shell • All are nonmetals • Very reactive are often bonded with elements from Group 1 http: //www. youtube. com/watch? v=u 2 og. MUDBaf 4&f eature=player_embedded Episode 402
Noble Gases Group 18 �Exist as gases �Non-metals � 8 electrons in the outer shell = Full �Helium (He) has only 2 electrons in the outer shell = Full �Not reactive with other elements Episode 402
Rare Earth Metals Lanthanides Actinides • Some are Radioactive • The rare earths are silver, silverywhite, or gray metals. • Conduct electricity Episode 402
- Slides: 17