Periodic Table Basic Structure Rows and Columns The

Periodic Table Basic Structure

Rows and Columns � The Periodic Table has • 7 rows called “Periods” – 1 -7 • 18 columns called “Groups” – 1 -18

Element Arrangement �Elements are ordered from left to right, top to bottom by increasing atomic number �Atomic numbers are 1 -118

Common Properties �Elements in each column, or group, have common chemical properties • Example: all elements in Group 18 are gases. • The collective name for this group of elements is the “Nobel Gases” • Other groups have names as well
Lots of Information �The Periodic Table can give us lots of information about the atoms of each type of element

Chemical Symbol �Each element (kind of atom) has a unique chemical symbol. �This is used as a short way of writing the names of elements �These symbols are also used when writing chemical equations �Each chemical symbol is made up of 1 -3 letters
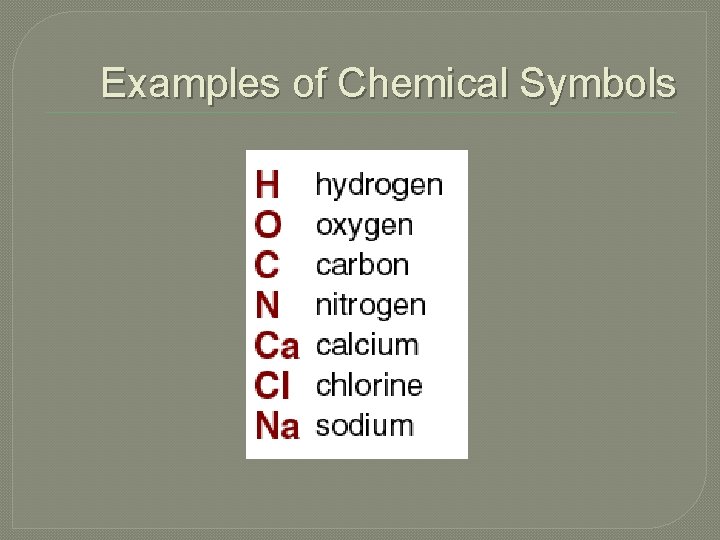
Examples of Chemical Symbols
Chemical Symbols Cont’d �The first letter of each chemical symbol is ALWAYS CAPITALIZED. �All other letters in the symbol are lowercase �Writing chemical symbols properly is very important in chemistry. �Capitalizing the wrong letter may indicate a different element.

Examples of Improper Capitalization of Chemical Symbols �Cl = Chlorine (correct) �CI = Carbon and Iodine (incorrect) �Co = Cobalt (correct) �CO = Carbon Monoxide (incorrect) �Si = Silicon (correct) �SI = Sulfur and Iodine (incorrect)
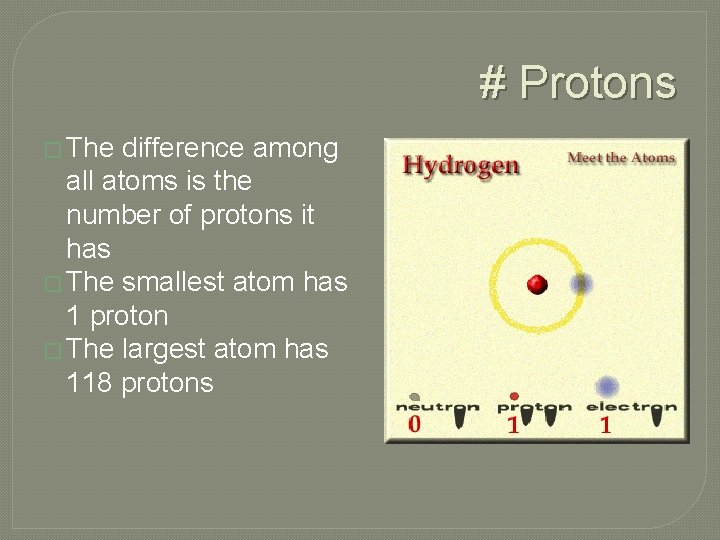
# Protons � The difference among all atoms is the number of protons it has � The smallest atom has 1 proton � The largest atom has 118 protons

Atomic Number �This indicates the number of protons in each atom of a particular element. �This number is usually in the largest font in each box. �Notice that atomic numbers increase as you look left to right across the Periodic Table
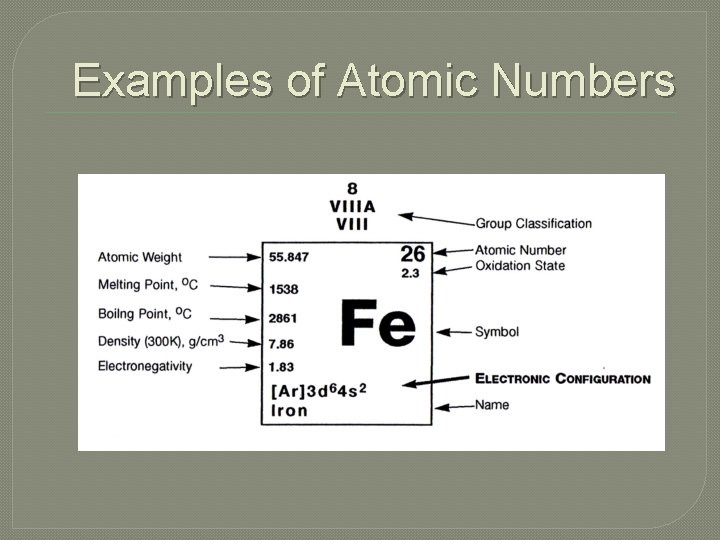
Examples of Atomic Numbers

Importance of Atomic Number �The identity of an atom is determined by it’s atomic number �Knowing the atomic number of an element can help you draw an electrically neutral atom for that element
- Slides: 13