Periodic Table and Ion Formation Atoms gain and
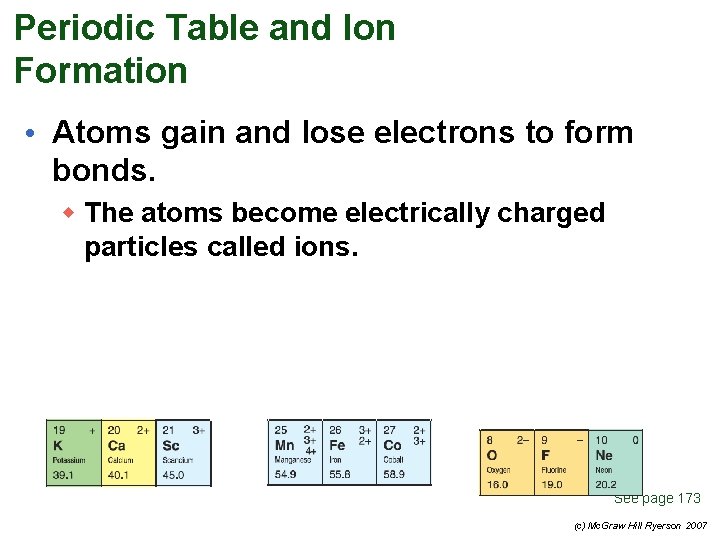
Periodic Table and Ion Formation • Atoms gain and lose electrons to form bonds. w The atoms become electrically charged particles called ions. See page 173 (c) Mc. Graw Hill Ryerson 2007
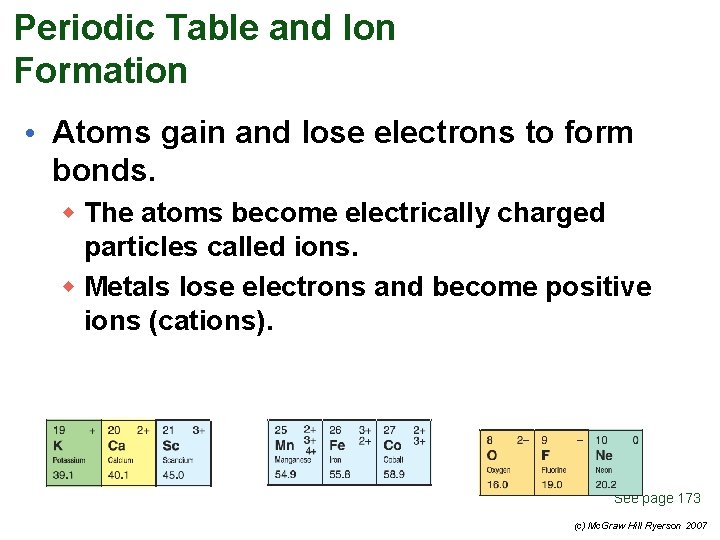
Periodic Table and Ion Formation • Atoms gain and lose electrons to form bonds. w The atoms become electrically charged particles called ions. w Metals lose electrons and become positive ions (cations). See page 173 (c) Mc. Graw Hill Ryerson 2007
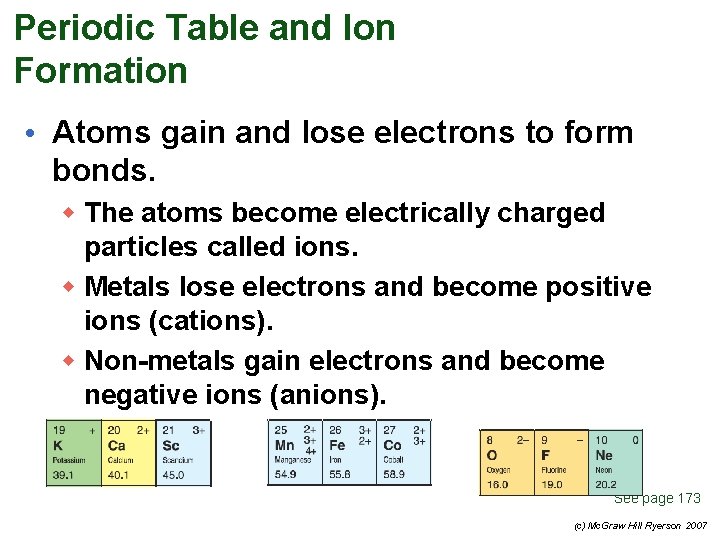
Periodic Table and Ion Formation • Atoms gain and lose electrons to form bonds. w The atoms become electrically charged particles called ions. w Metals lose electrons and become positive ions (cations). w Non-metals gain electrons and become negative ions (anions). See page 173 (c) Mc. Graw Hill Ryerson 2007
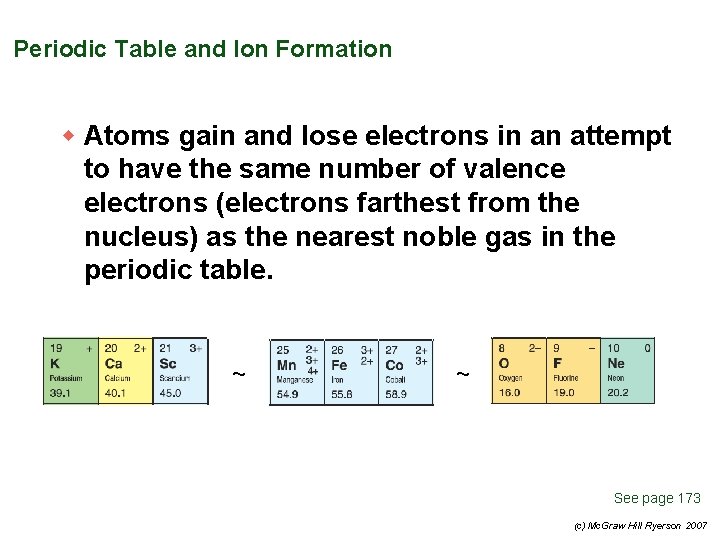
Periodic Table and Ion Formation w Atoms gain and lose electrons in an attempt to have the same number of valence electrons (electrons farthest from the nucleus) as the nearest noble gas in the periodic table. ~ ~ See page 173 (c) Mc. Graw Hill Ryerson 2007
Forming Compounds ATOMIC BONDING See pages 176 - 177 (c) Mc. Graw Hill Ryerson 2007

Forming Compounds • When two atoms get close together, their valence electrons interact. See pages 176 - 177 (c) Mc. Graw Hill Ryerson 2007
Forming Compounds • When two atoms get close together, their valence electrons interact. w If the valence electrons can combine to form a lowenergy bond, a compound is formed. See pages 176 - 177 (c) Mc. Graw Hill Ryerson 2007

Forming Compounds • When two atoms get close together, their valence electrons interact. w If the valence electrons can combine to form a lowenergy bond, a compound is formed. w Each atom in the compound attempts to have the stable number of valence electrons as the nearest noble gas. See pages 176 - 177 (c) Mc. Graw Hill Ryerson 2007
Forming Compounds • When two atoms get close together, their valence electrons interact. w If the valence electrons can combine to form a lowenergy bond, a compound is formed. w Each atom in the compound attempts to have the stable number of valence electrons as the nearest noble gas. w Metals may lose electrons and non-metals may gain electrons to form an IONIC BOND (IONIC BONDS ONLY OCCUR BETWEEN METALS AND NON-METALS) See pages 176 - 177 (c) Mc. Graw Hill Ryerson 2007

Forming Compounds • Ionic bonds form when electrons are transferred from positive ions to negative ions. See pages 176 - 177 (c) Mc. Graw Hill Ryerson 2007
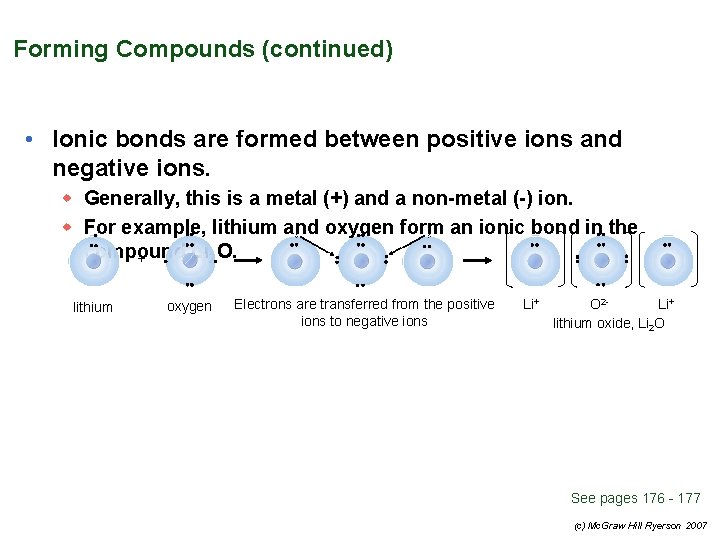
Forming Compounds (continued) • Ionic bonds are formed between positive ions and negative ions. w Generally, this is a metal (+) and a non-metal (-) ion. w For example, lithium and oxygen form an ionic bond in the compound Li 2 O. + lithium oxygen Electrons are transferred from the positive ions to negative ions Li+ O 2 Li+ lithium oxide, Li 2 O See pages 176 - 177 (c) Mc. Graw Hill Ryerson 2007

EXAMPLE (c) Mc. Graw Hill Ryerson 2007
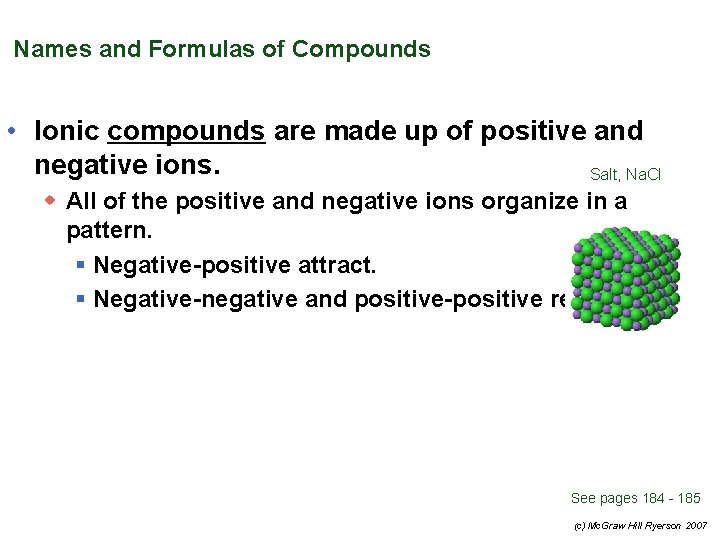
Names and Formulas of Compounds • Ionic compounds are made up of positive and negative ions. Salt, Na. Cl w All of the positive and negative ions organize in a pattern. § Negative-positive attract. § Negative-negative and positive-positive repel. See pages 184 - 185 (c) Mc. Graw Hill Ryerson 2007
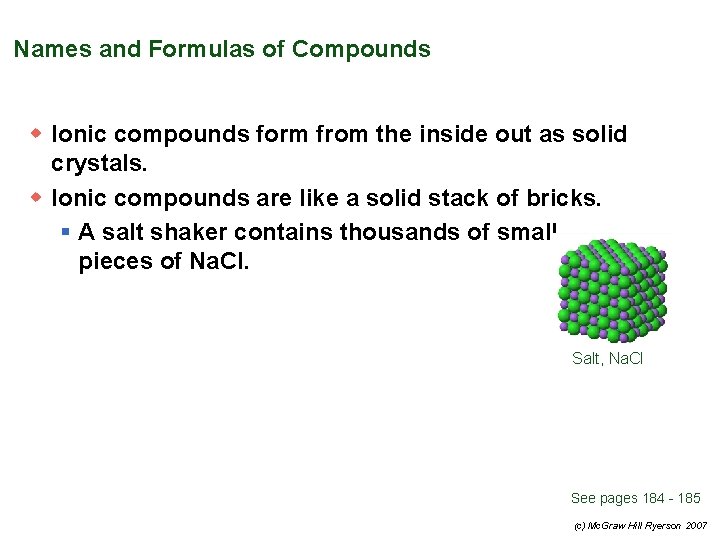
Names and Formulas of Compounds w Ionic compounds form from the inside out as solid crystals. w Ionic compounds are like a solid stack of bricks. § A salt shaker contains thousands of small pieces of Na. Cl. Salt, Na. Cl See pages 184 - 185 (c) Mc. Graw Hill Ryerson 2007

The Chemical Name and Formula of an Ionic Compound w The name of an ionic compound = positive ion + negative ion-ide. w For example, an ionic compound forms between magnesium and oxygen. § The positive ion is the first part of the name, magnesium. § The negative ion forms part of the ending of the name, ox(ygen). § Add -ide to the end of the name to form magnesium Magnesium oxide is used oxide. as a drying agent. See pages 186 - 187 (c) Mc. Graw Hill Ryerson 2007
The Chemical Name and Formula of an Ionic Compound • Ionic formulas are based on the ions of the atoms involved. w Remember the naming principles above. w For example, what is the name of Ca 3 N 2? § Ca, the positive ion, is calcium. § N, the negative ion, is nitrogen. § Drop the end of the anion and add -ide. Magnesium oxide is used as a drying agent. See pages 186 - 187 (c) Mc. Graw Hill Ryerson 2007
The Chemical Name and Formula of an Ionic Compound • Ionic formulas are based on the ions of the atoms involved. w Remember the naming principles above. w For example, what is the name of Ca 3 N 2? § Ca, the positive ion, is calcium. § N, the negative ion, is nitrogen. § Drop the end of the anion and add -ide. § Calcium nitride Magnesium oxide is used as a drying agent. See pages 186 - 187 (c) Mc. Graw Hill Ryerson 2007

The Chemical Name and Formula of an Ionic Compound (continued) • Writing formulas for ionic compounds: w In an ionic compound, the positive charges balance out the negative charges. w ***ELECTRONS ARE TRANSFERRED FROM ATOMS. THEY CAN’T BE CREATED OR DESTROYED!**** Calcium oxide, also known as “quicklime” was once produced by cooking limestone in ancient kilns. See page 188 (c) Mc. Graw Hill Ryerson 2007

The Chemical Name and Formula of an Ionic Compound (continued) • Writing formulas for ionic compounds: w In an ionic compound, the positive charges balance out the negative charges. w ***ELECTRONS ARE TRANSFERRED FROM ATOMS. THEY CAN’T BE CREATED OR DESTROYED!**** w The ratio of positive: negative charges gives the proper formula. oxide, also § The ratio is always written in Calcium reduced known as form. “quicklime” was once produced by cooking limestone in ancient kilns. See page 188 (c) Mc. Graw Hill Ryerson 2007

The Chemical Name and Formula of an Ionic Compound (continued) w For example, what is the formula for magnesium phosphide? Calcium oxide, also known as “quicklime” was once produced by cooking limestone in ancient kilns. See page 188 (c) Mc. Graw Hill Ryerson 2007

The Chemical Name and Formula of an Ionic Compound (continued) w For example, what is the formula for magnesium phosphide? 2+ 3– § magnesium is Mg phosphorous is P Calcium oxide, also known as “quicklime” was once produced by cooking limestone in ancient kilns. See page 188 (c) Mc. Graw Hill Ryerson 2007
The Chemical Name and Formula of an Ionic Compound (continued) w For example, what is the formula for magnesium phosphide? 2+ 3– § magnesium is Mg phosphorous is P Calcium oxide, also § Lowest common multiple of 2 and 3 “quicklime” isknown 6 aswas once produced by cooking limestone in ancient kilns. See page 188 (c) Mc. Graw Hill Ryerson 2007
The Chemical Name and Formula of an Ionic Compound (continued) w For example, what is the formula for magnesium phosphide? 2+ 3– § magnesium is Mg phosphorous is P Calcium oxide, also § Lowest common multiple of 2 and 3 “quicklime” isknown 6 aswas once produced by § 3 Mg 2+ ions and 2 P 3– ions cooking limestone in ancient kilns. See page 188 (c) Mc. Graw Hill Ryerson 2007
The Chemical Name and Formula of an Ionic Compound (continued) w For example, what is the formula for magnesium phosphide? 2+ 3– § magnesium is Mg phosphorous is P Calcium oxide, also § Lowest common multiple of 2 and 3 “quicklime” isknown 6 aswas once produced by § 3 Mg 2+ ions and 2 P 3– ions cooking limestone in ancient kilns. § Mg 3 P 2 See page 188 (c) Mc. Graw Hill Ryerson 2007

The Chemical Name and Formula of an Ionic Compound (continued) w Try the formula for calcium oxide. Calcium oxide, also known as “quicklime” was once produced by cooking limestone in ancient kilns. See page 188 (c) Mc. Graw Hill Ryerson 2007
The Chemical Name and Formula of an Ionic Compound (continued) w Try the formula for calcium oxide. § calcium is Ca 2+ oxygen is O 2– § Lowest common multiple of 2 and 2 is 2 § 1 Ca 2+ ion and 1 O 2– ions § Ca. O Calcium oxide, also known as “quicklime” was once produced by cooking limestone in ancient kilns. See page 188 (c) Mc. Graw Hill Ryerson 2007
- Slides: 26