Periodic Properties Trends in the periodic table of

Periodic Properties Trends in the periodic table of the elements
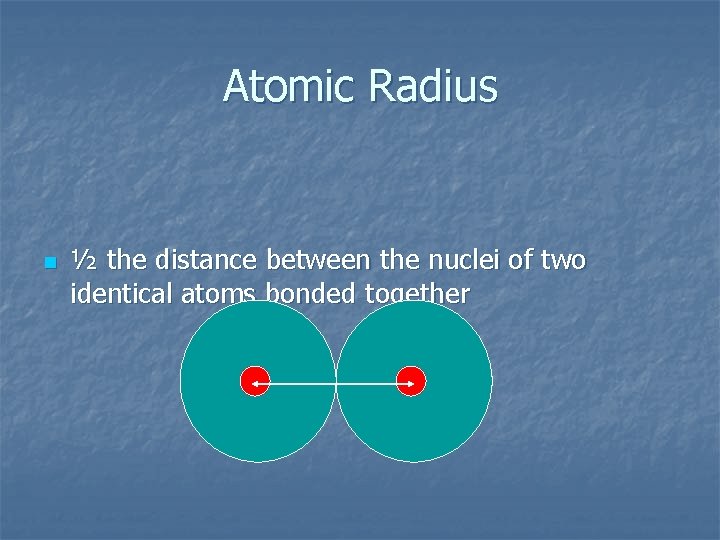
Atomic Radius n ½ the distance between the nuclei of two identical atoms bonded together
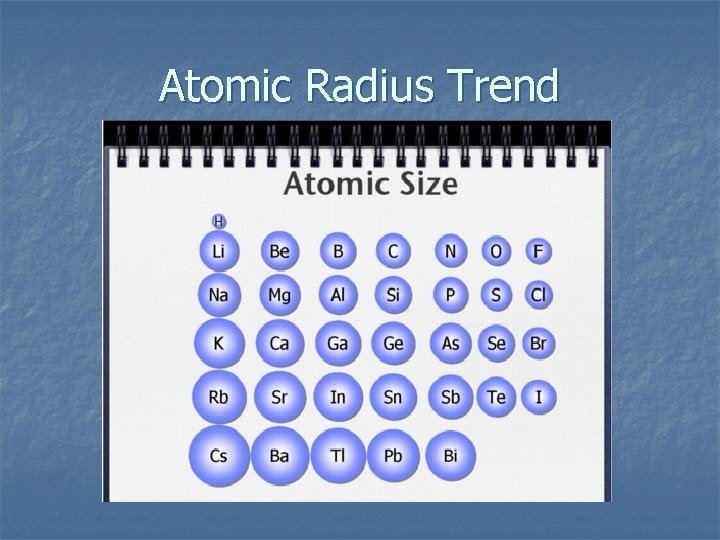
Atomic Radius Trend
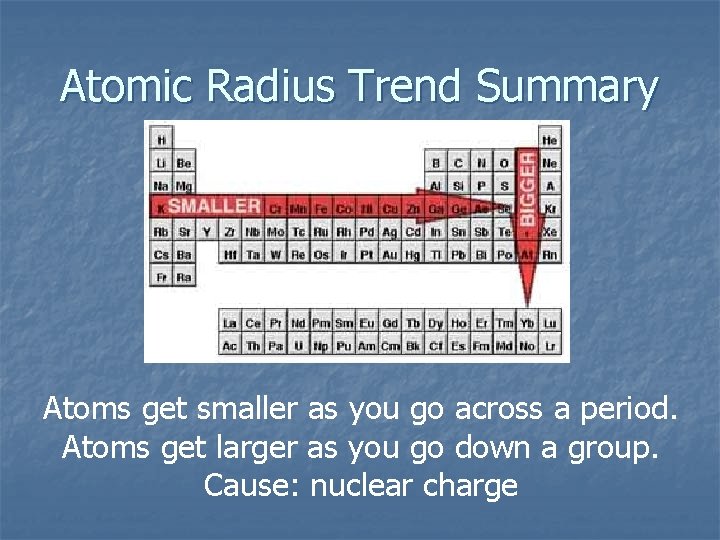
Atomic Radius Trend Summary Atoms get smaller as you go across a period. Atoms get larger as you go down a group. Cause: nuclear charge

Check Yourself: n n n Which of the following elements has the smallest atomic radius? A. Be B. Ca C. N D. Fe

Ionization Energy n The energy required to remove an electron from a neutral atom 1 st ionization energy – to remove one en 2 nd ionization energy – to remove a 2 nd en 3 rd ionization energy – to remove a 3 rd e. And so on… n
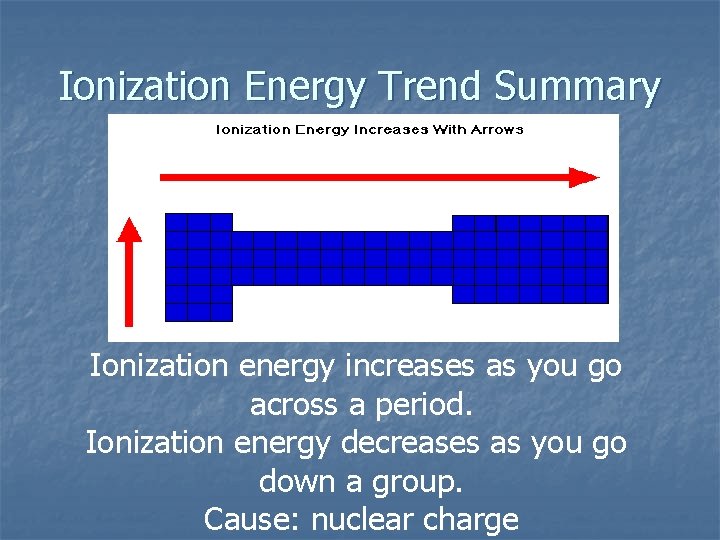
Ionization Energy Trend Summary Ionization energy increases as you go across a period. Ionization energy decreases as you go down a group. Cause: nuclear charge

Check Yourself: n n Which elements are the most reactive metals? Why? Which elements are the most reactive nonmetals? Why?
Ionic Radius n An ion is bonded to a reference ion and the radius is measured
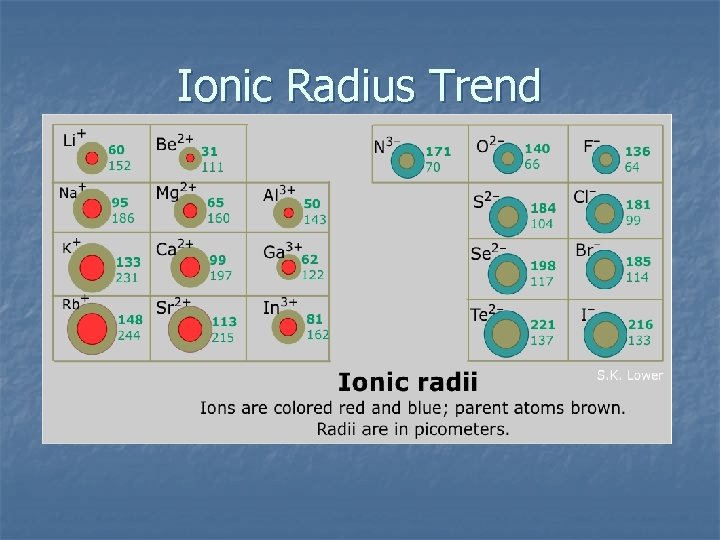
Ionic Radius Trend

Ionic Radius Trend Summary n n Cations form when elements lose e-. Positive ions are smaller than their parent atom. Anions form when elements gain e-. Negative ions are larger than their parent atom. Cause: nuclear charge
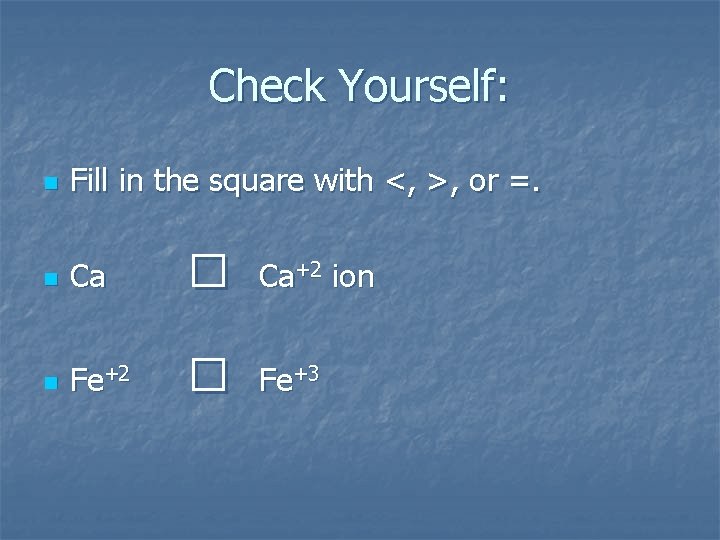
Check Yourself: n Fill in the square with <, >, or =. n Ca n Fe+2 □ □ Ca+2 ion Fe+3

Electronegativity The ability of an element in a chemical bond to attract electrons. An element’s “need” for another electron. Values of 0 (lowest) to 4 (highest) on the Pauling scale
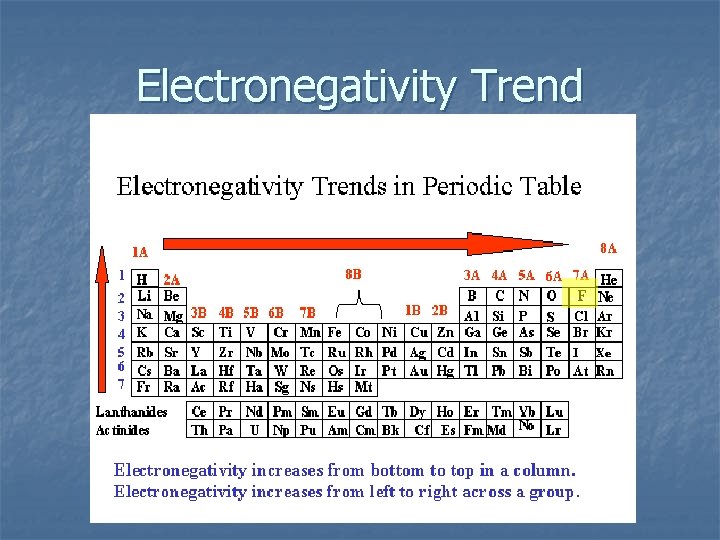
Electronegativity Trend ….
Periodic Trends Summary Atomic Radius Ionic Radius
- Slides: 15