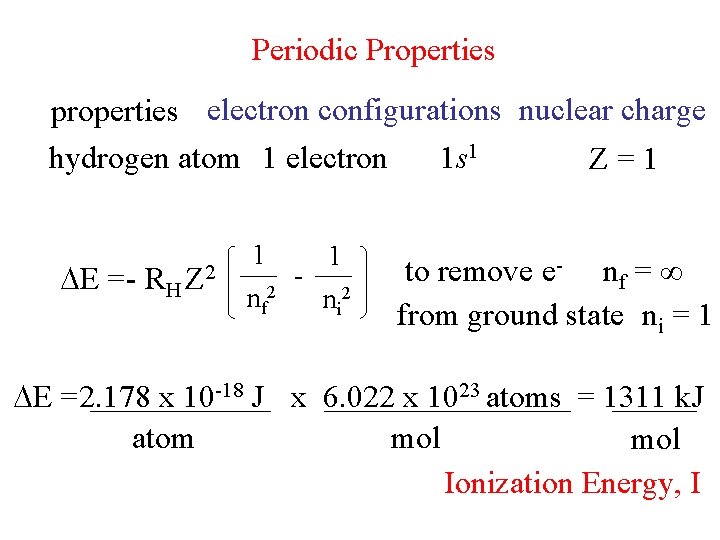
Periodic Properties properties electron configurations nuclear charge hydrogen
- Slides: 14

Periodic Properties properties electron configurations nuclear charge hydrogen atom 1 electron 1 s 1 Z=1 E = - RH Z 2 1 nf 2 - 1 ni 2 to remove e- nf = ∞ from ground state ni = 1 E =2. 178 x 10 -18 J x 6. 022 x 1023 atoms = 1311 k. J atom mol Ionization Energy, I

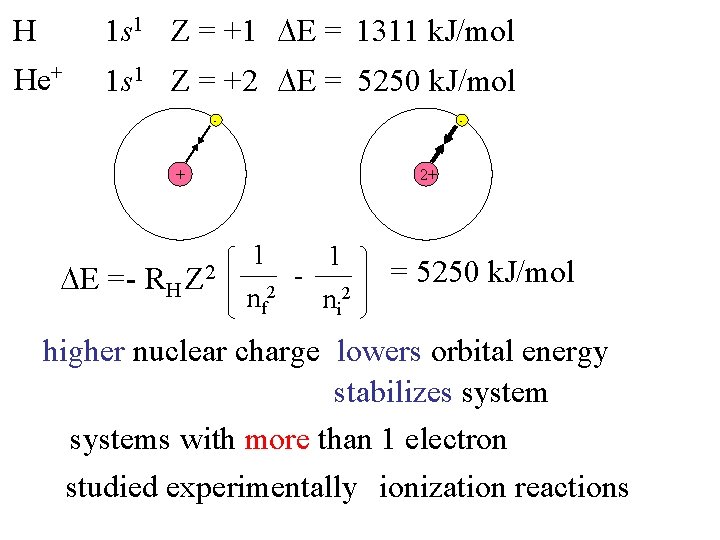
H 1 s 1 Z = +1 E = 1311 k. J/mol He+ 1 s 1 Z = +2 E = 5250 k. J/mol - - + E = - RH 2+ Z 2 1 nf 2 - 1 ni 2 = 5250 k. J/mol higher nuclear charge lowers orbital energy stabilizes systems with more than 1 electron studied experimentally ionization reactions

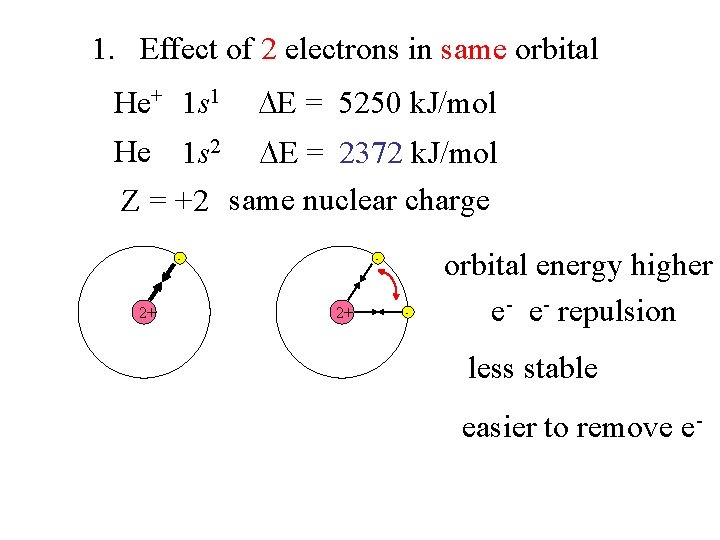
1. Effect of 2 electrons in same orbital He+ 1 s 1 E = 5250 k. J/mol He 1 s 2 E = 2372 k. J/mol Z = +2 same nuclear charge - 2+ - orbital energy higher e- e- repulsion less stable easier to remove e-

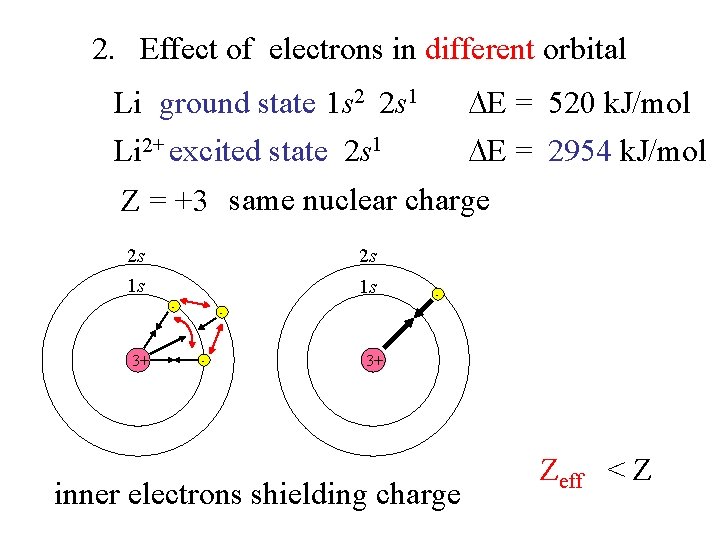
2. Effect of electrons in different orbital Li ground state 1 s 2 2 s 1 E = 520 k. J/mol Li 2+ excited state 2 s 1 E = 2954 k. J/mol Z = +3 same nuclear charge 2 s 2 s 1 s 1 s - 3+ - - - 3+ inner electrons shielding charge Zeff < Z

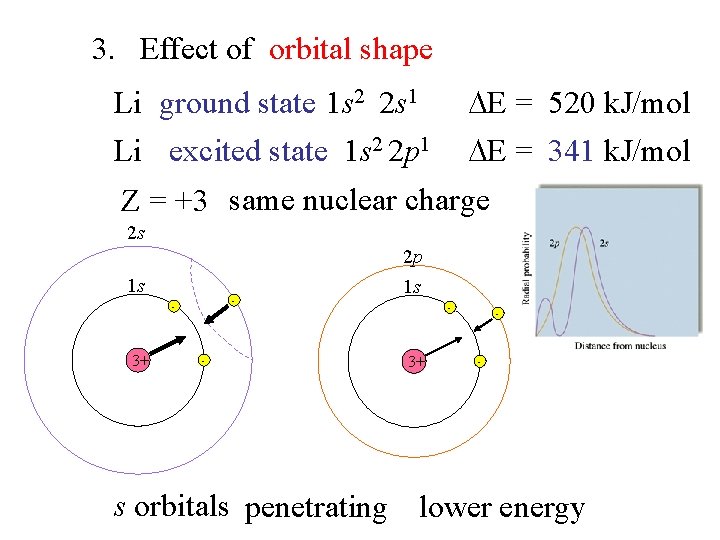
3. Effect of orbital shape Li ground state 1 s 2 2 s 1 E = 520 k. J/mol Li excited state 1 s 2 2 p 1 E = 341 k. J/mol Z = +3 same nuclear charge 2 s 2 p 1 s - - 3+ - 1 s - 3+ - - s orbitals penetrating lower energy

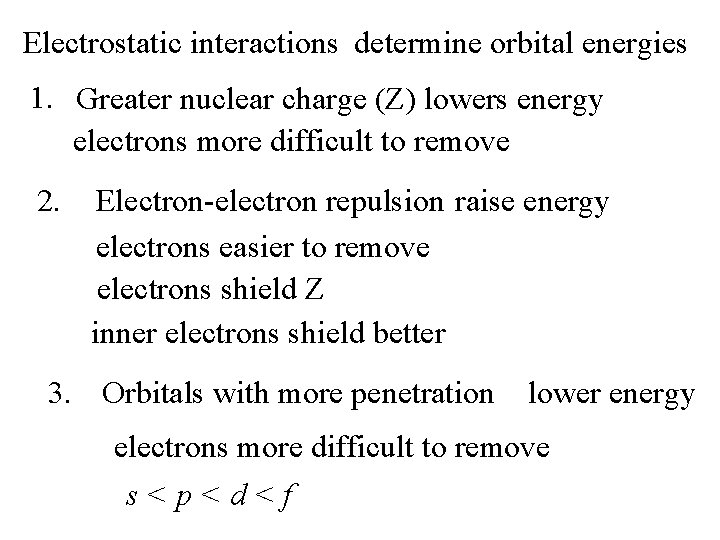
Electrostatic interactions determine orbital energies 1. Greater nuclear charge (Z) lowers energy electrons more difficult to remove 2. Electron-electron repulsion raise energy electrons easier to remove electrons shield Z inner electrons shield better 3. Orbitals with more penetration lower energy electrons more difficult to remove s<p<d<f

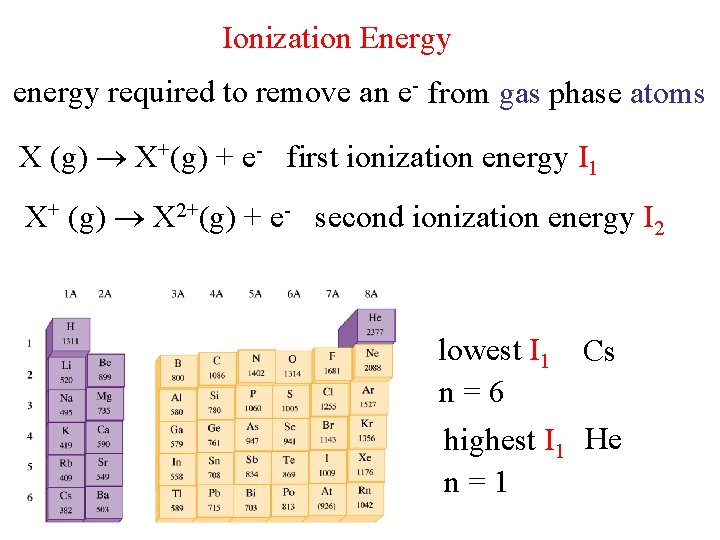
Ionization Energy energy required to remove an e- from gas phase atoms X (g) X+(g) + e- first ionization energy I 1 X+ (g) X 2+(g) + e- second ionization energy I 2 lowest I 1 Cs n=6 highest I 1 He n=1

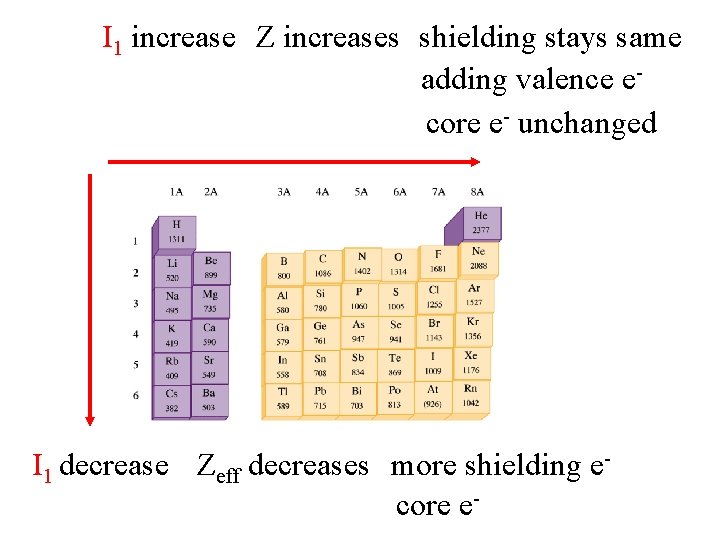
I 1 increase Z increases shielding stays same adding valence ecore e- unchanged I 1 decrease Zeff decreases more shielding ecore e-

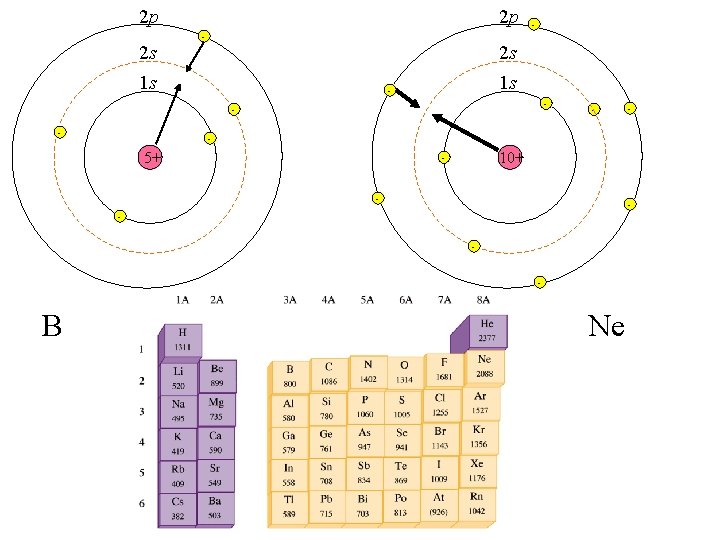
2 p 2 s 2 p - - 2 s 1 s 1 s - - - 5+ 10+ - - B - Ne

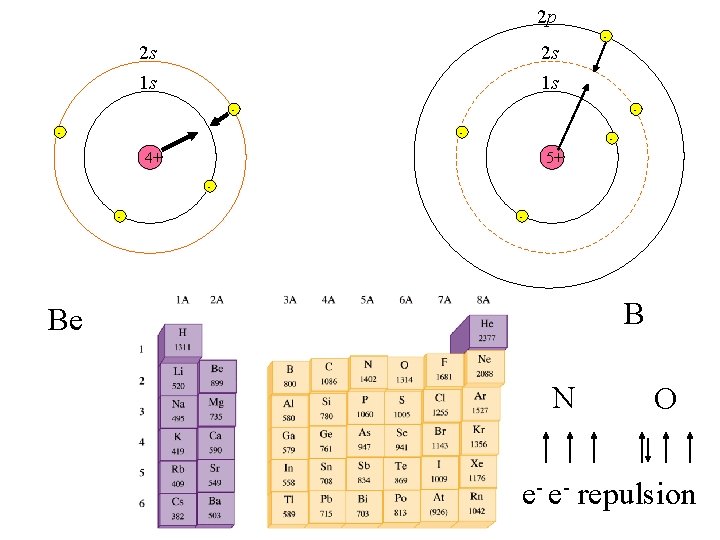
2 p 2 s 2 s 1 s 1 s - - 4+ 5+ - - - B Be N O e- e- repulsion

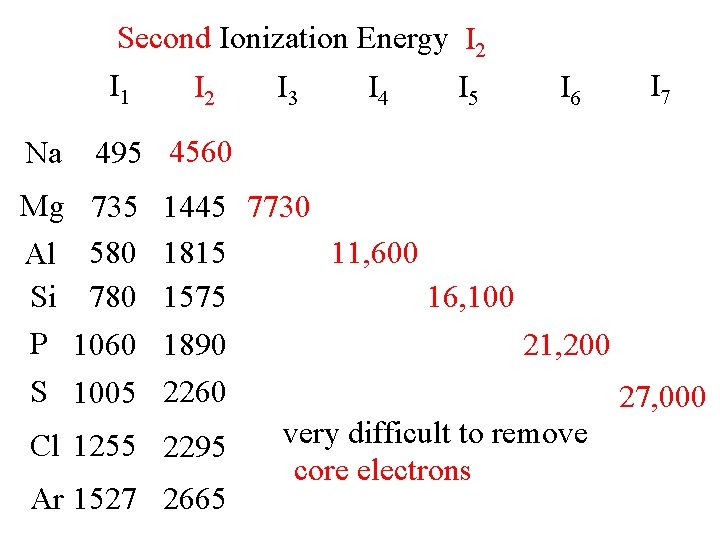
Second Ionization Energy I 2 I 1 I 2 I 3 I 4 I 5 Na I 6 I 7 495 4560 Mg 735 Al 580 Si 780 P 1060 S 1005 1445 7730 1815 11, 600 1575 16, 100 1890 21, 200 2260 27, 000 very difficult to remove Cl 1255 2295 core electrons Ar 1527 2665

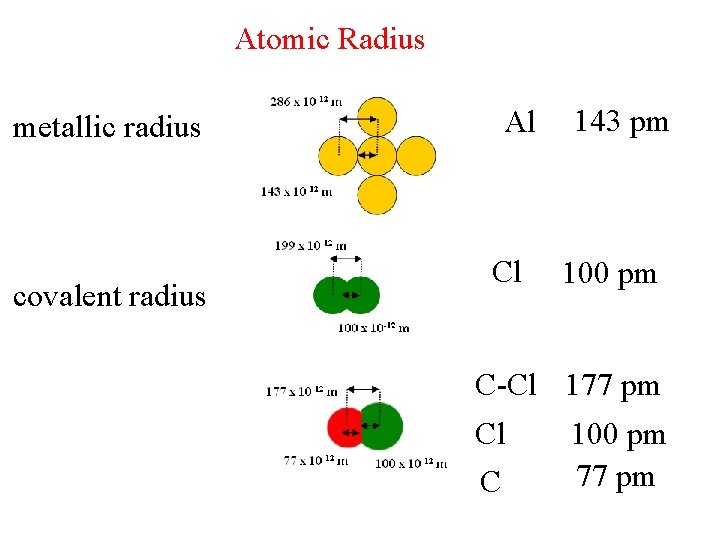
Atomic Radius metallic radius covalent radius Al Cl 143 pm 100 pm C-Cl 177 pm Cl C 100 pm 77 pm

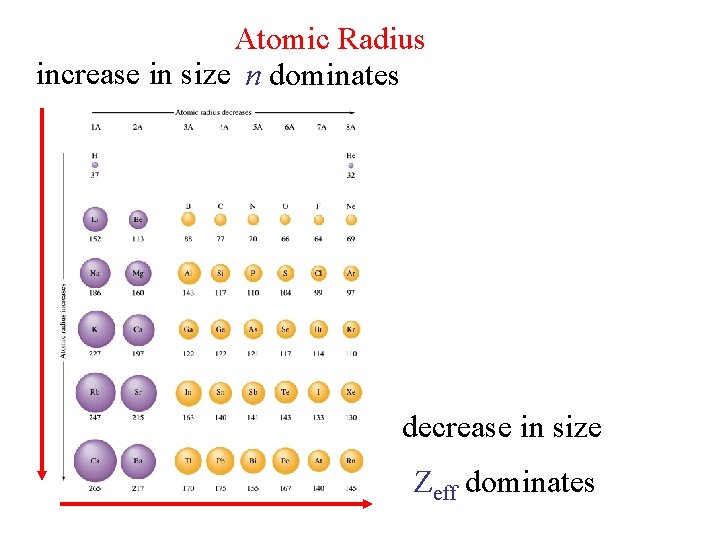
Atomic Radius increase in size n dominates decrease in size Zeff dominates

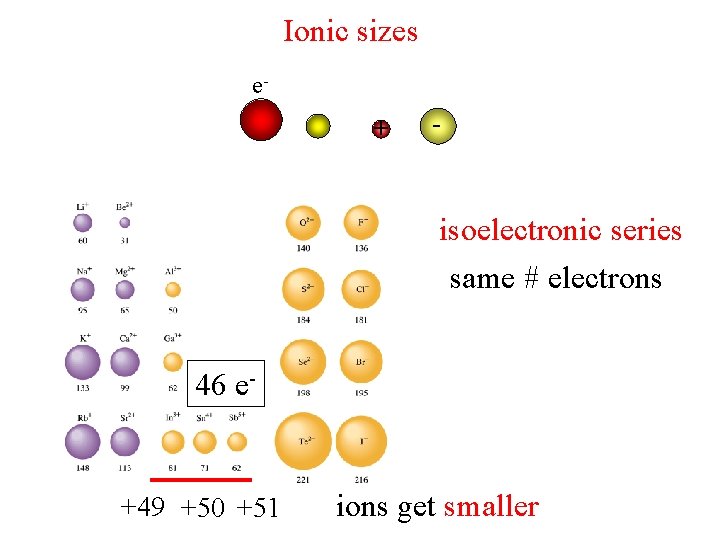
Ionic sizes e+ - isoelectronic series same # electrons 46 e- +49 +50 +51 ions get smaller