Periodic Properties of the Elements How Orbitals Fill
Periodic Properties of the Elements How Orbitals Fill The Basis of the Periodic Table Trends in Atomic Properties of Elements

Mendeleev is given credit for the Periodic Table

The following slides we shall skip through fast because. . . • . . . they were stolen from the internet and • I cannot find their source. • But the information is useful!

The History of the Modern Periodic Table

During the nineteenth century, chemists began to categorize the elements according to similarities in their physical and chemical properties. The end result of these studies was our modern periodic table.
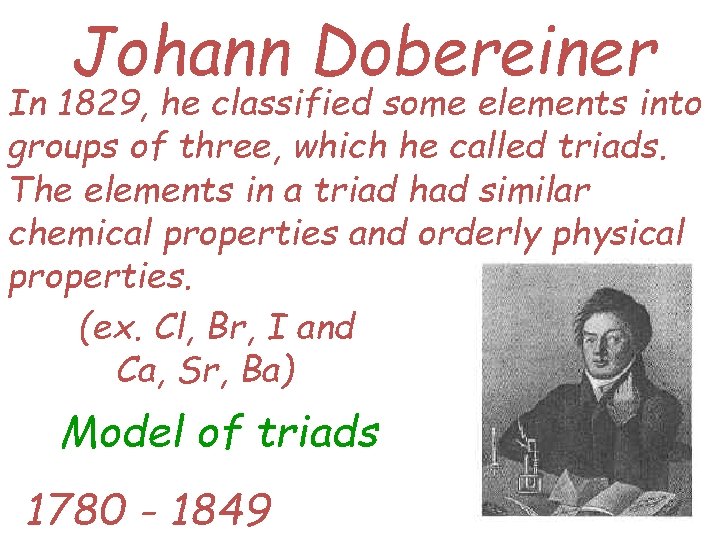
Johann Dobereiner In 1829, he classified some elements into groups of three, which he called triads. The elements in a triad had similar chemical properties and orderly physical properties. (ex. Cl, Br, I and Ca, Sr, Ba) Model of triads 1780 - 1849
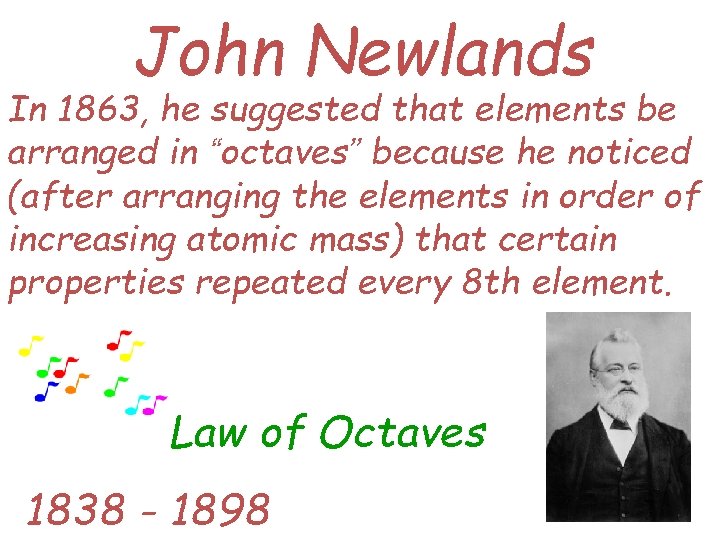
John Newlands In 1863, he suggested that elements be arranged in “octaves” because he noticed (after arranging the elements in order of increasing atomic mass) that certain properties repeated every 8 th element. Law of Octaves 1838 - 1898
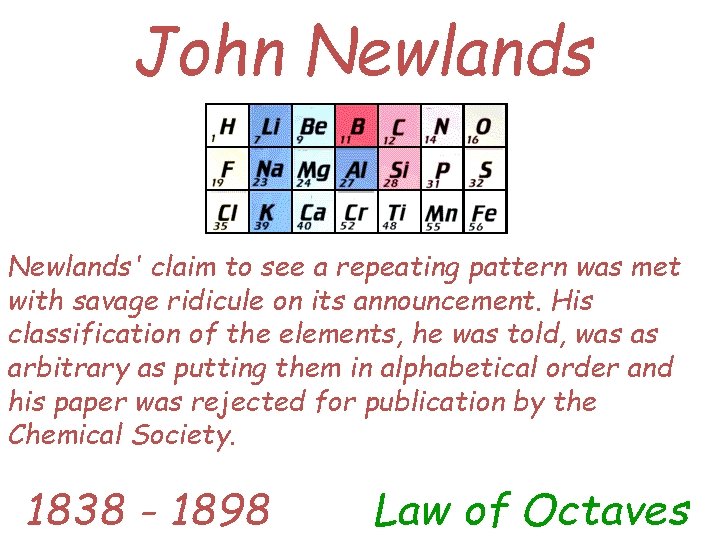
John Newlands' claim to see a repeating pattern was met with savage ridicule on its announcement. His classification of the elements, he was told, was as arbitrary as putting them in alphabetical order and his paper was rejected for publication by the Chemical Society. 1838 - 1898 Law of Octaves

John Newlands His law of octaves failed beyond the WHY? element calcium. Would his law of octaves work today with the first 20 elements? 1838 - 1898 Law of Octaves

Dmitri Mendeleev In 1869 he published a table of the elements organized by increasing atomic mass. 1834 - 1907

Lothar Meyer At the same time, he published his own table of the elements organized by increasing atomic mass. 1830 - 1895

• Both Mendeleev and Meyer arranged the elements in order of increasing atomic mass. • Both left vacant spaces where unknown elements should fit. So why is Mendeleev called the “father of the modern periodic table” and not Meyer, or both?

Mendeleev. . . • stated that if the atomic weight of an element caused it to be placed in the wrong group, then the weight must be wrong. (He corrected the atomic masses of Be, In, and U) • was so confident in his table that he used it to predict the physical properties of three elements that were yet unknown.

After the discovery of these unknown elements between 1874 and 1885, and the fact that Mendeleev’s predictions for Sc, Ga, and Ge were amazingly close to the actual values, his table was generally accepted.

However, in spite of Mendeleev’s great achievement, problems arose when new elements were discovered and more accurate atomic weights determined. By looking at our modern periodic table, can you identify what problems might have caused chemists a headache? Ar and K Co and Ni Te and I Th and Pa

Henry Moseley In 1913, through his work with X-rays, he determined the actual nuclear charge (atomic number) of the elements*. He rearranged the elements in order of increasing atomic number. *“There is in the atom a fundamental quantity which increases by regular steps as we pass from each element to the next. This quantity can only be the charge on the central positive nucleus. ” 1887 - 1915

Henry Moseley His research was halted when the British government sent him to serve as a foot soldier in WWI. He was killed in the fighting in Gallipoli by a sniper’s bullet, at the age of 28. Because of this loss, the British government later restricted its scientists to noncombatant duties during WWII.

Glenn T. Seaborg After co-discovering 10 new elements, in 1944 he moved 14 elements out of the main body of the periodic table to their current location below the Lanthanide series. These became known as the Actinide series. 1912 - 1999

Glenn T. Seaborg He is the only person to have an element named after him while still alive. "This is the greatest honor ever bestowed upon me - even better, I think, than winning the Nobel Prize. " 1912 - 1999
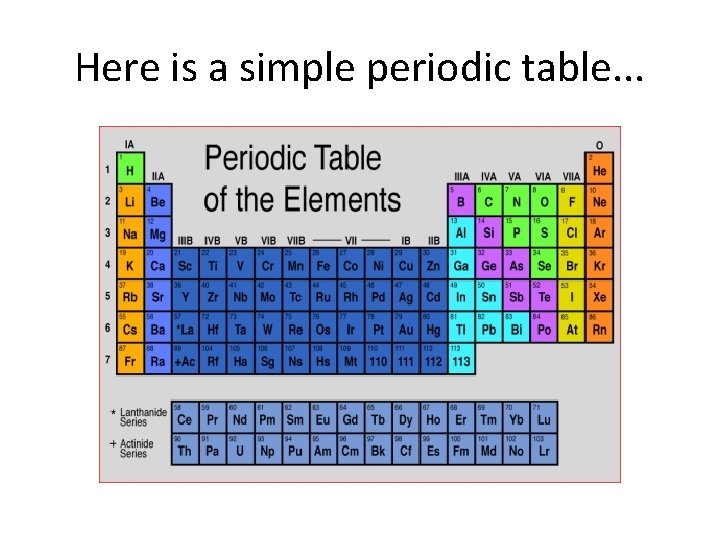
Here is a simple periodic table. . .
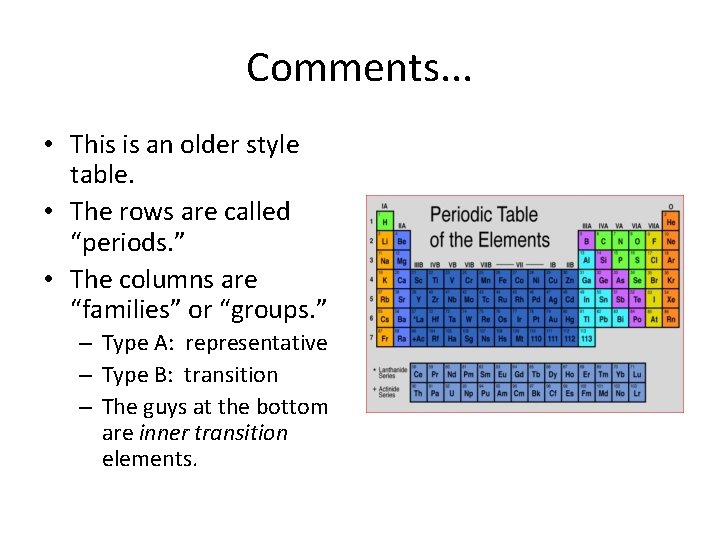
Comments. . . • This is an older style table. • The rows are called “periods. ” • The columns are “families” or “groups. ” – Type A: representative – Type B: transition – The guys at the bottom are inner transition elements.

Elements in Columns Have Similar Properties • This is especially true of type A (representative) elements. • But, there are changes in reactivity, etc. , as one goes down a column. • This link gives group IA as an example. . . http: //www. youtube. com/watch? v=Ft 4 E 1 e. CUIt. I • More about vertical trends later!
Looking at the rows. . . • The rows are called periods. • How they fill gives insight into the atomic structures. • In particular, we shall examine the idea of electron configurations.
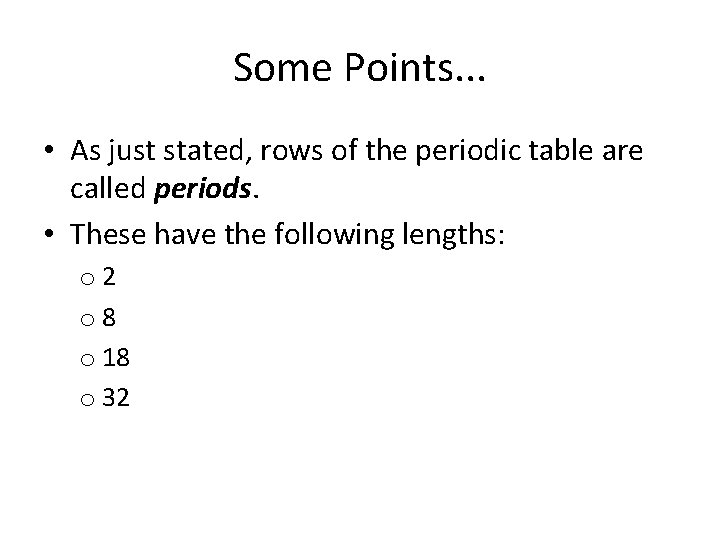
Some Points. . . • As just stated, rows of the periodic table are called periods. • These have the following lengths: o 2 o 8 o 18 o 32

Look at these numbers in detail. . . • • 2 = 2 x 12 8 = 2 x 22 18 = 2 x 32 32 = 2 x 42 • This gives some insight into quantum numbers!

In the last chapter we saw. . . • 3 quantum numbers, n, l, and ml. • For each value of n we have n 2 orbitals. • We play some number games here: vn = 1 → 1 orbital vn = 2 → 4 orbitals (4 = 1 + 3) vn = 3 → 9 orbitals (9 = 1 + 3 + 5) vn = 4 → 16 orbitals (16 = 1 + 3 + 5 + 7) • These are all ½ the lengths of the periods! What’s wrong?

Solution to this problem. . . • H has just one electron. • The Schrödinger equation seen earlier needed another quantum number to handle multielectron atoms. (Technical detail: This comes naturally if relativity included. ) • This new quantum number is ms, the electron spin. This has values of = +1/2 and -1/2. • The physical explanation is left for lecture!
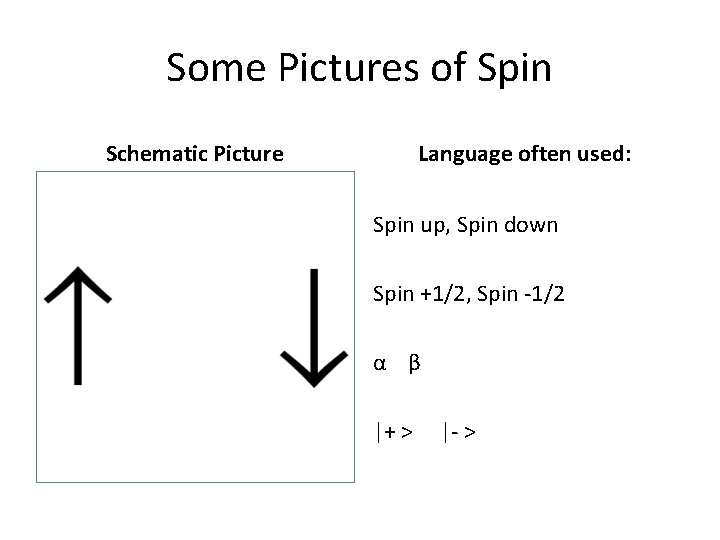
Some Pictures of Spin Schematic Picture Language often used: Spin up, Spin down Spin +1/2, Spin -1/2 α β |+ > |- >
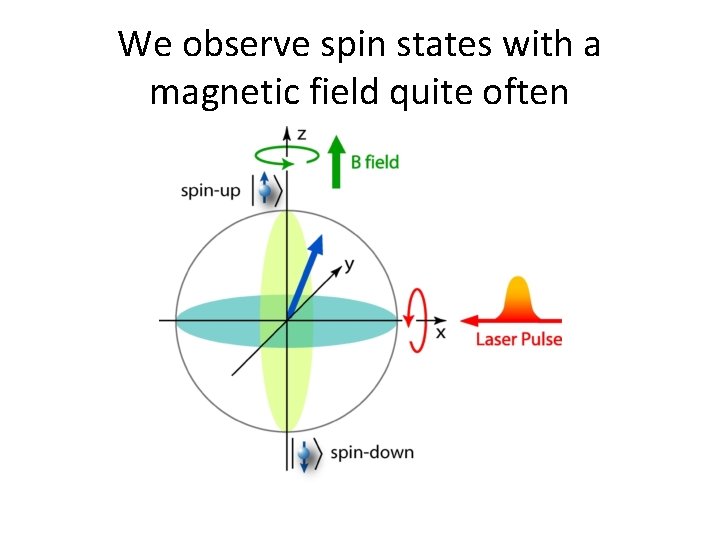
We observe spin states with a magnetic field quite often
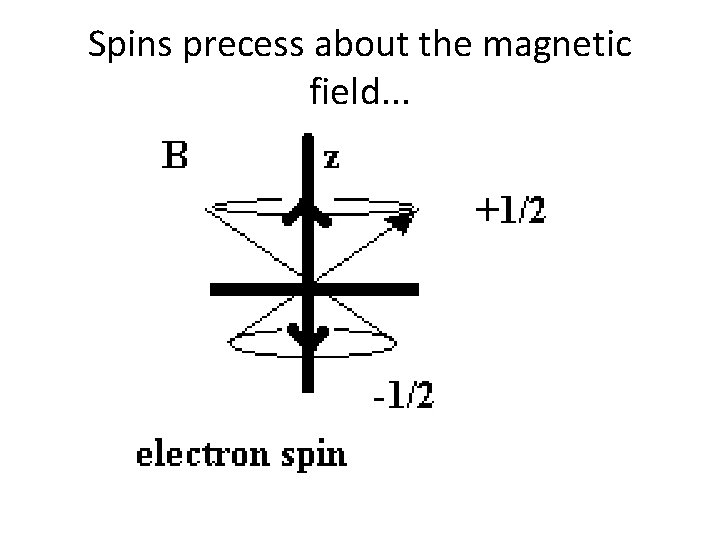
Spins precess about the magnetic field. . .
Other depictions of spin
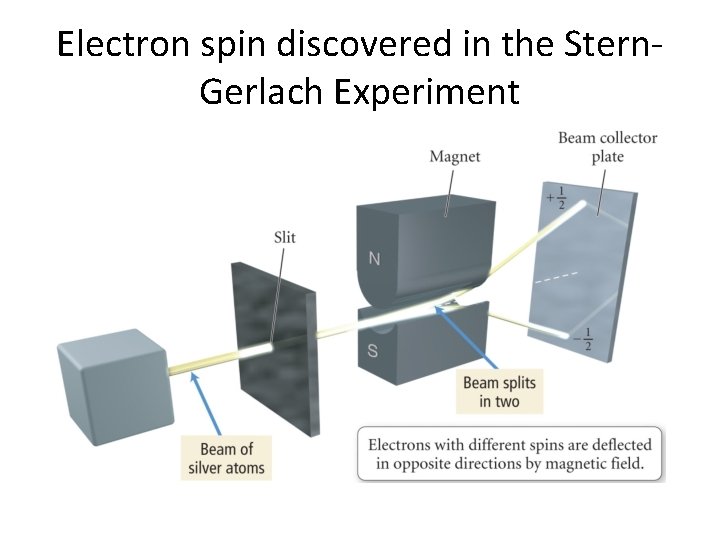
Electron spin discovered in the Stern. Gerlach Experiment

The Guys. . . Otto Stern Walter Gerlach
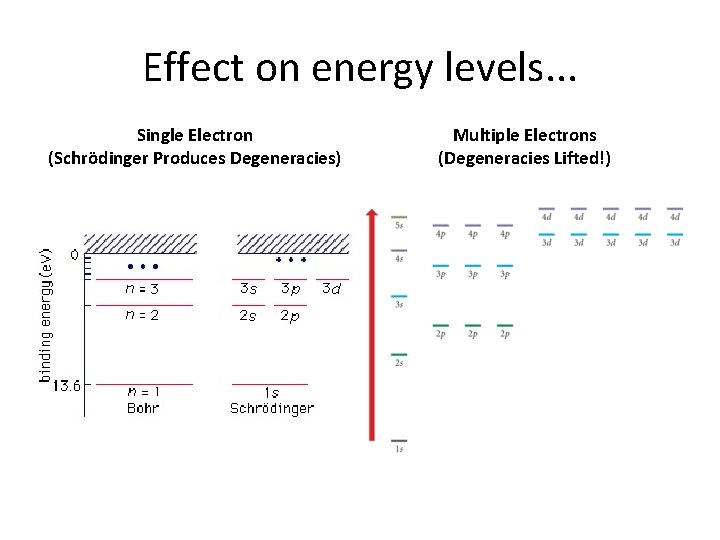
Effect on energy levels. . . Single Electron (Schrödinger Produces Degeneracies) Multiple Electrons (Degeneracies Lifted!)
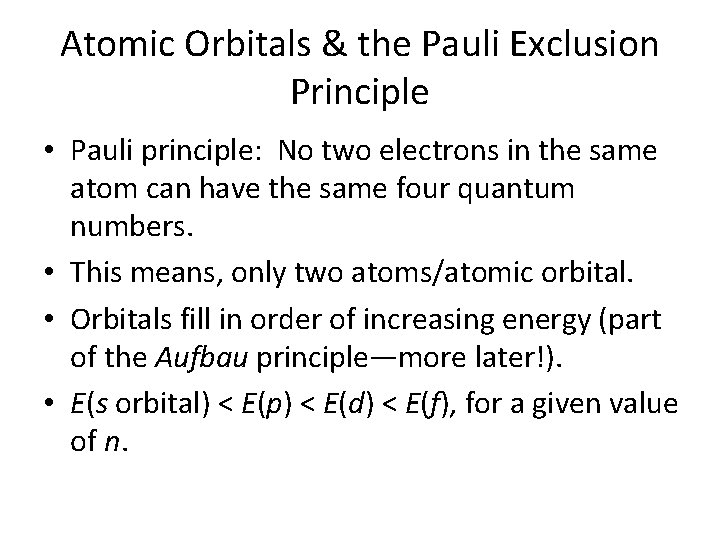
Atomic Orbitals & the Pauli Exclusion Principle • Pauli principle: No two electrons in the same atom can have the same four quantum numbers. • This means, only two atoms/atomic orbital. • Orbitals fill in order of increasing energy (part of the Aufbau principle—more later!). • E(s orbital) < E(p) < E(d) < E(f), for a given value of n.

Some more guys. . . Wolfgang Pauli & Bohr Studying Spin
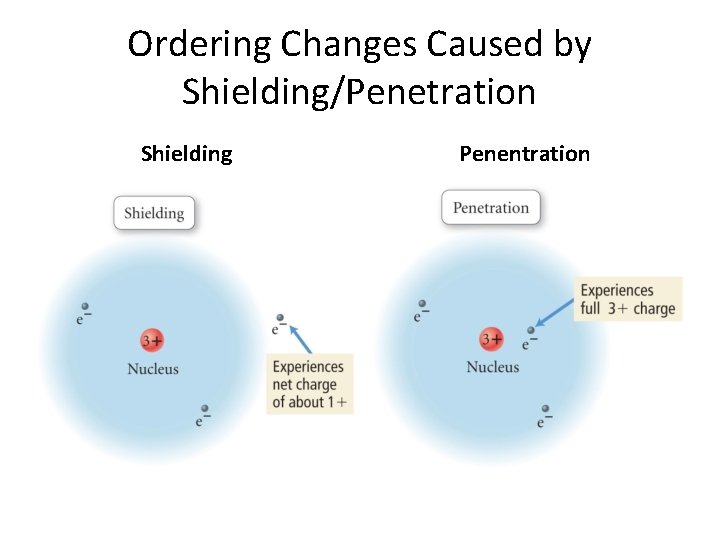
Ordering Changes Caused by Shielding/Penetration Shielding Penentration
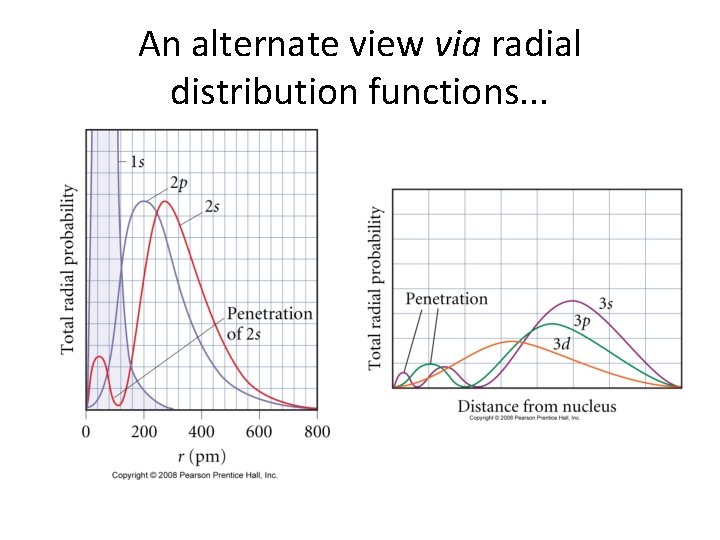
An alternate view via radial distribution functions. . .

Orbital Filling: The Aufbau Principle Niels Bohr Friedrich Hund

The Aufbau Principle • Electrons fill atoms in order of increasing orbital energy. q 1 s 2 s 2 p 3 s 3 p 4 s 3 d 4 p 5 s 4 d 5 p 6 s, etc. • Degenerate orbitals fill so as to maximize the number of parallel electron spins. q. This is Hund’s rule!
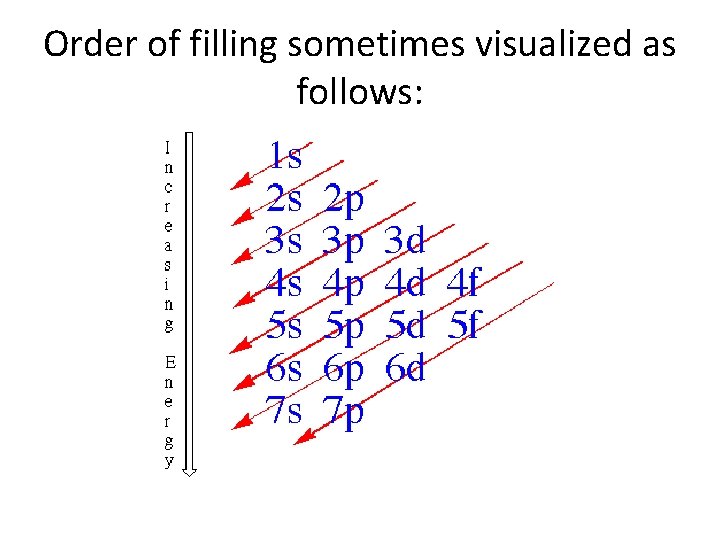
Order of filling sometimes visualized as follows:
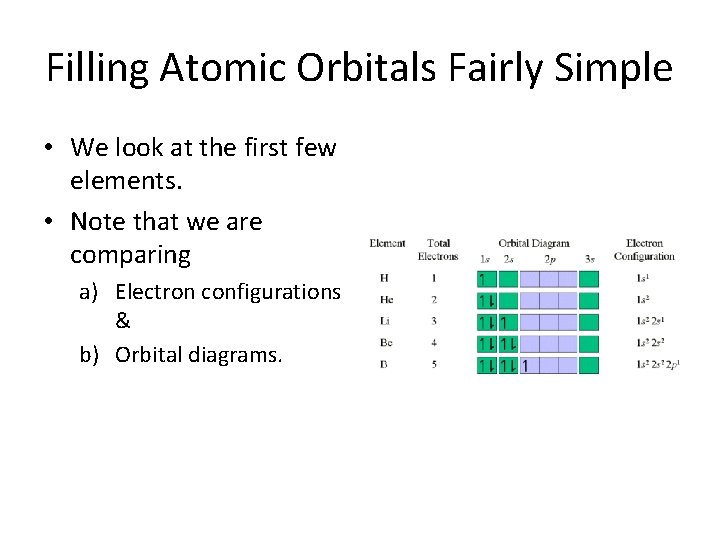
Filling Atomic Orbitals Fairly Simple • We look at the first few elements. • Note that we are comparing a) Electron configurations & b) Orbital diagrams.
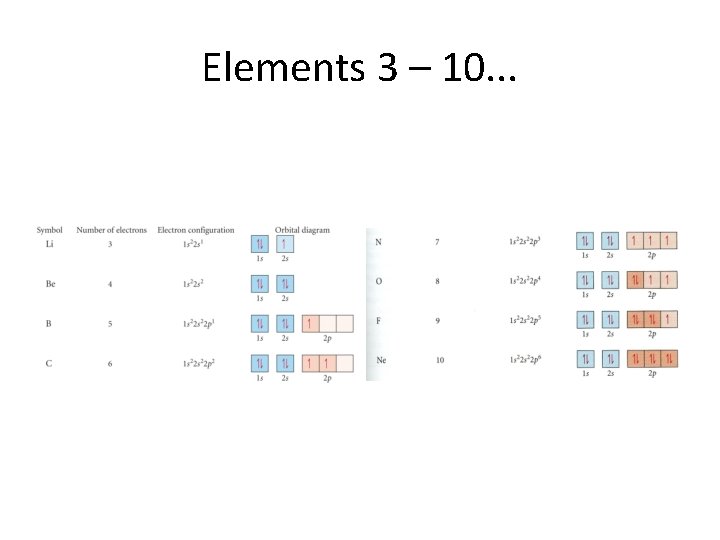
Elements 3 – 10. . .
Notational Shortcut • We often write in CORE electrons as their INERT GAS SHELLS. • Examples: – Na as [Ne]3 s 1 instead of 1 s 22 p 63 s 1 – Rb as [Kr]5 s 1 instead of a really long mess! • We see the first 18 elements to the right. . .
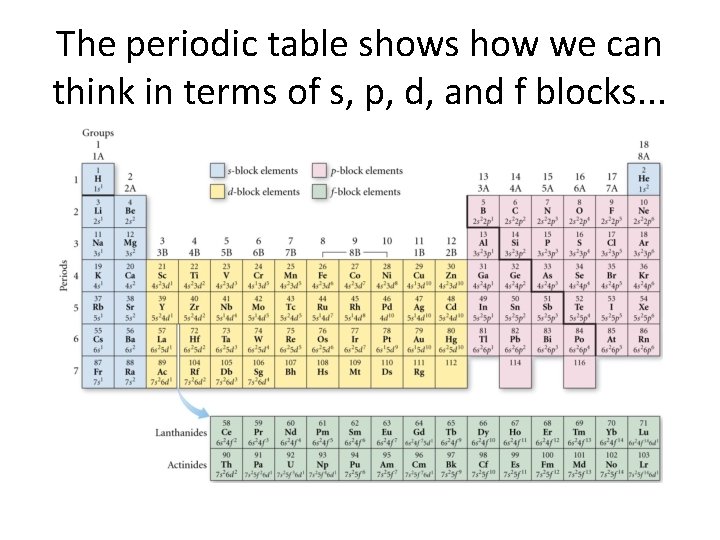
The periodic table shows how we can think in terms of s, p, d, and f blocks. . .

One thing should be obvious. . . You can construct an electron configuration directly from the periodic table (usually)!
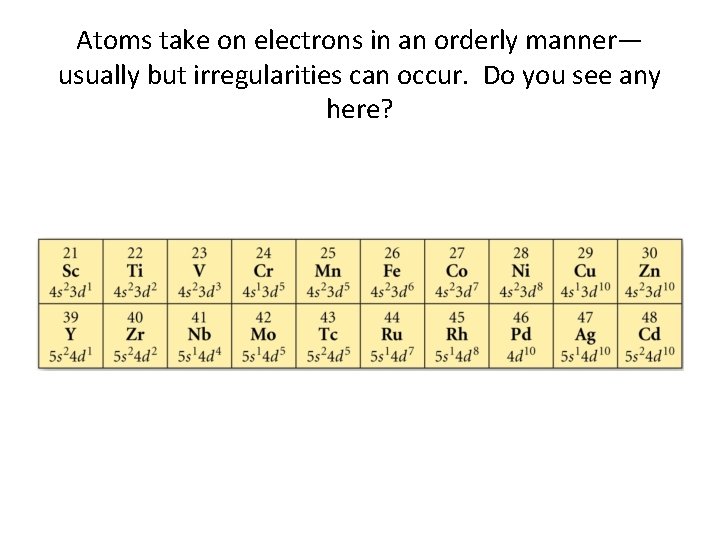
Atoms take on electrons in an orderly manner— usually but irregularities can occur. Do you see any here?

A corollary that follows from Hund’s rule & Aufbau. . . • Half-filled subshells are particularly stable. • Fully-filled subshells are particular stable. • What appears to be an anomaly really is not!

Atoms-first Viewpoint Here. . . • Electronic configurations build atoms. • Atoms with similar configurations are similar in properties. • The periodic table and configurations are intimately related. • In the next few slides, we shall show a few of these happy families of elements.
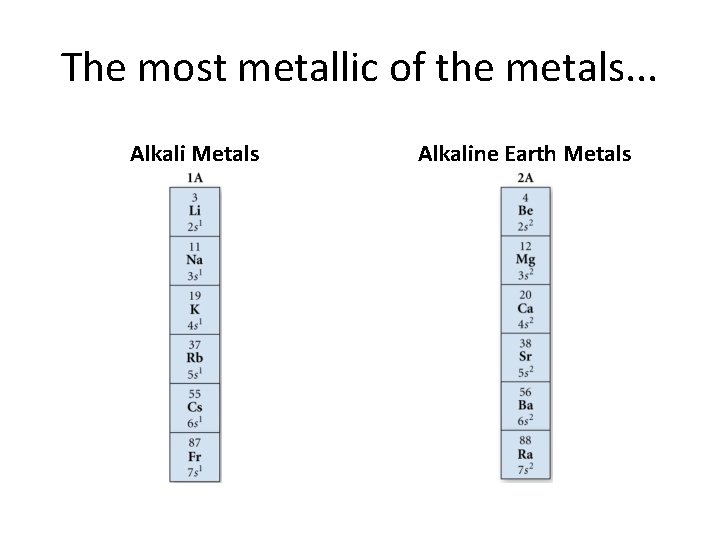
The most metallic of the metals. . . Alkali Metals Alkaline Earth Metals
The most noxious and snobbiest elements. . . The Halogens The Noble Metals
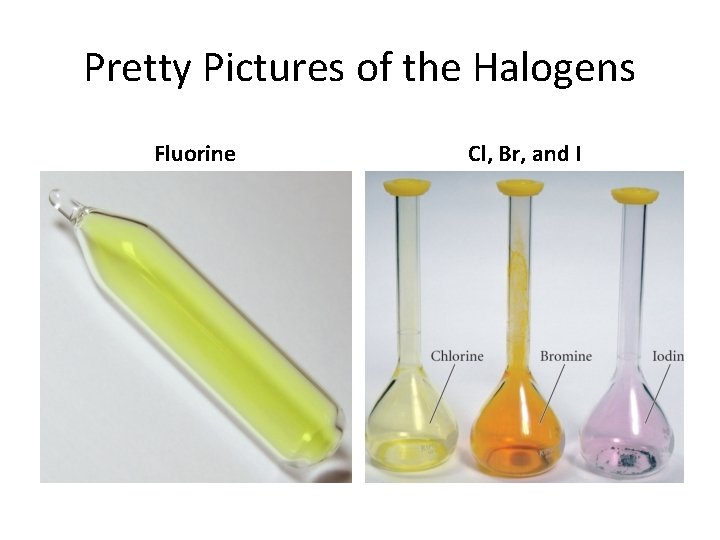
Pretty Pictures of the Halogens Fluorine Cl, Br, and I

One last family • The last family we list is the chalcogens. • Sometimes, oxygen is left out of the group. • The group portrait is to the right. . .

The black sheep of the families • The elements in the 2 nd row of the periodic table often have quite different properties than the ones below them. • The elements to watch here are Li, Be, B, C, N, O, and F. • Why this happens will be explained later. But, for now: Beware of the 2 nd row!

Notable things about the exceptions. . . • Li reacts directly with N 2. No other element does. • Be is a deadly poison. Mg and Ca are nontoxic and the others are mildly toxic in this family. • B, N, C, and O can form double and triple bonds. Elements below them cannot. • F does not form any ions other than F-. (Cl, for instance, forms Cl. O-, Cl. O 2 -, Cl. O 3 -, and Cl. O 4 -. )
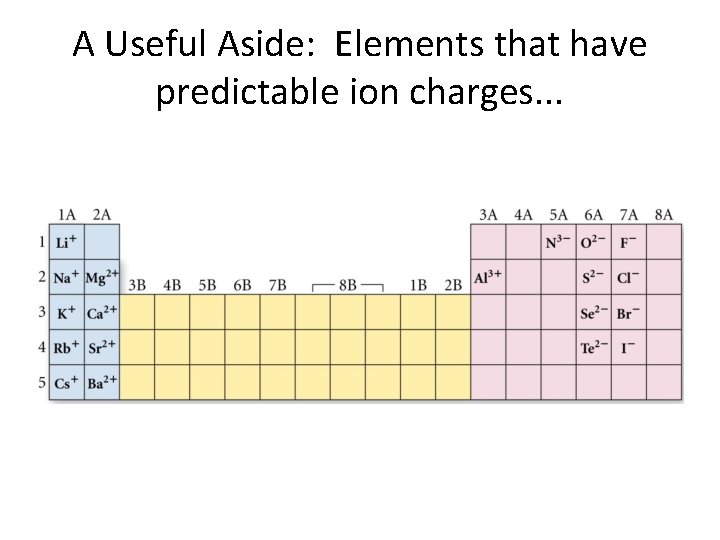
A Useful Aside: Elements that have predictable ion charges. . .

Enough about groups! Let us now discuss PERIODS!!!

Trends across periods. . . • We go let to right. • Here are some things we shall examine: – Atomic size (radii) – Ion size – Ionization energies – Electron affinities – Metallic Character • Trends are generally smooth but there are “bumps” to discuss!

Atom Size (Atomic radius) Definitions of atomic size: Nonbonding atomic radius (van der Waals) Bonding atomic radius Nonmetals: One-half the distance for two identical atoms bonded together Metals: One-half the distance between nuclei of two touching atoms in the metal crystal
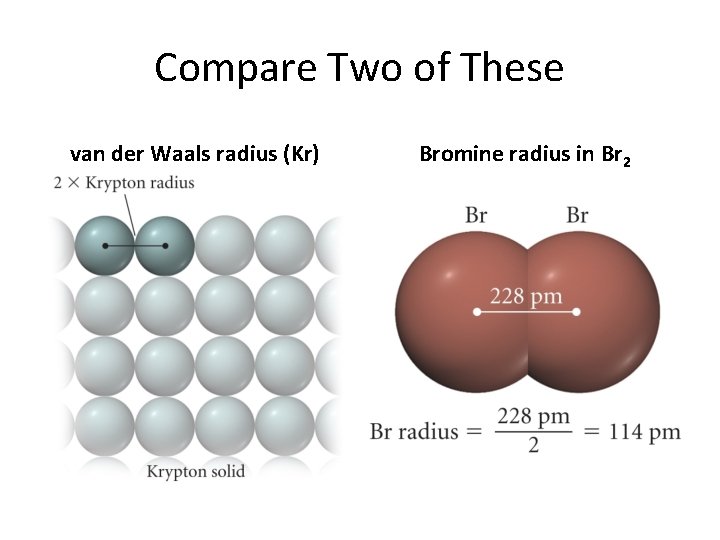
Compare Two of These van der Waals radius (Kr) Bromine radius in Br 2

Trends in Atomic Radius • A slight surprise: Atoms get smaller from left to right in a period! • But, there a few bumps. • There is NO surprise with columns. Things get bigger as you go down! (Example: Li < Na < K < Rb < Cs) • We shall discuss these things verbally as the next two slide are presented.
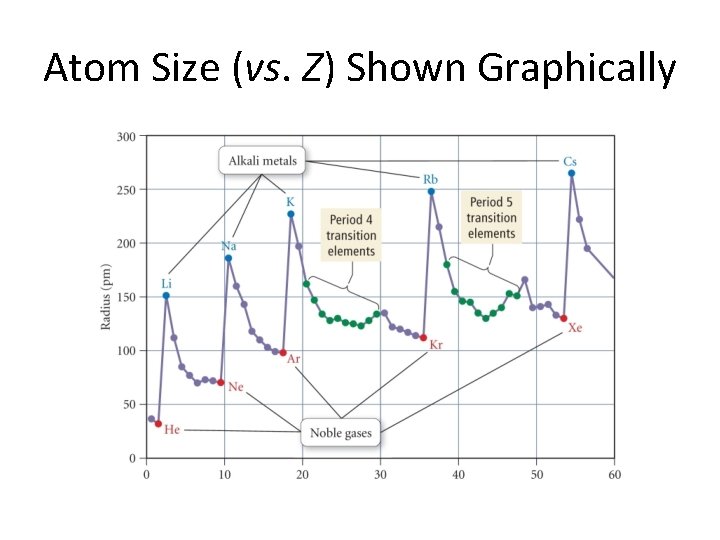
Atom Size (vs. Z) Shown Graphically
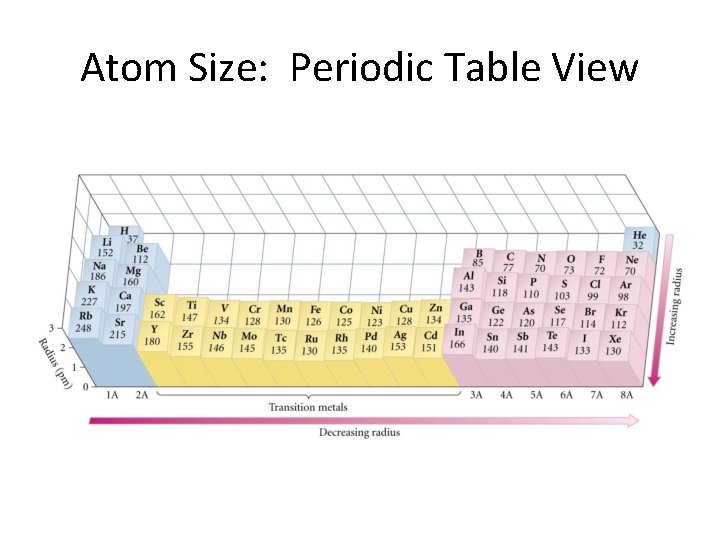
Atom Size: Periodic Table View

Causes of these trends. . . • As we move down a column in the periodic table, the principal quantum number of the electrons increases resulting in larger orbitals and, hence, larger atomic radii. • As we move to the right across a row, the effective nuclear charge experienced by the outermost electrons increases (for a given orbital) and, hence, the radius shrinks. (Zeff = Z – s, where s is the screening constant. )

Let’s Look at Monatomic Ions • Cations are positive (atoms lose electrons) • Anions are negative (atoms gain electrons) • Most ions form for one of the following reasons: – Getting an inert gas core – Attaining a half-filled d- or f-subshell – Attaining a fully-filled d- or f-subshell
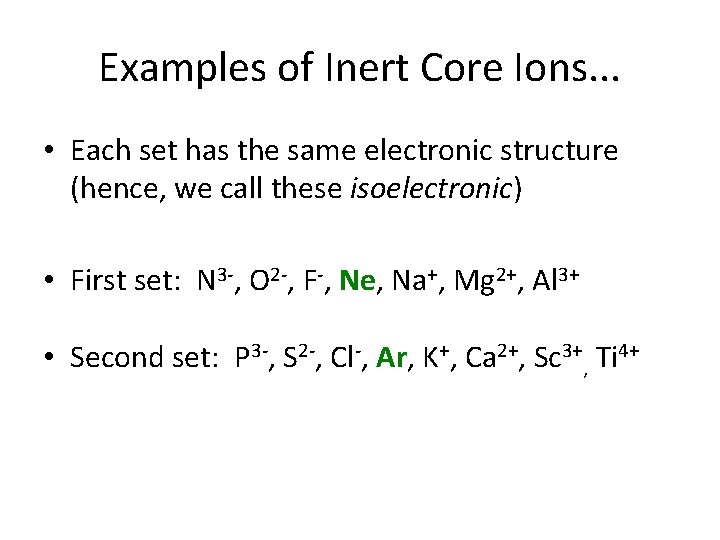
Examples of Inert Core Ions. . . • Each set has the same electronic structure (hence, we call these isoelectronic) • First set: N 3 -, O 2 -, F-, Ne, Na+, Mg 2+, Al 3+ • Second set: P 3 -, S 2 -, Cl-, Ar, K+, Ca 2+, Sc 3+, Ti 4+
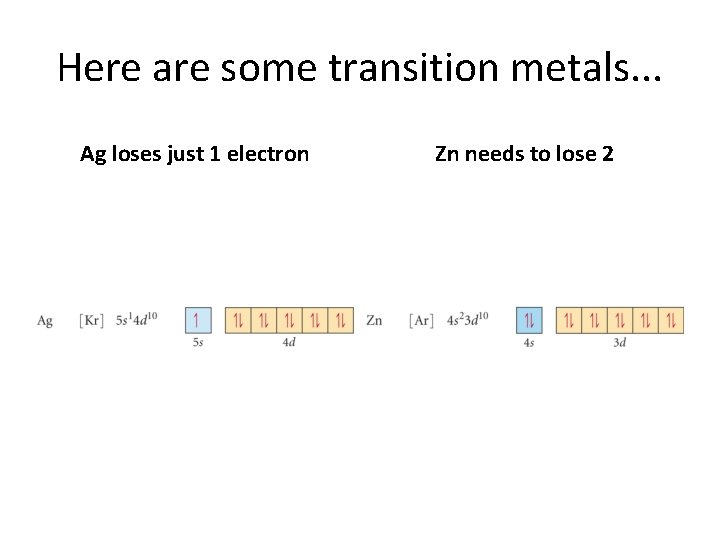
Here are some transition metals. . . Ag loses just 1 electron Zn needs to lose 2
Some At the Board Examples. . . Diamagnetism Paramagnetism • Electrons all paired. • Weakly repelled by magnet. • Do Al 3+ and S 2 - as examples. • One or more electrons unpaired (either an odd number or caused by Hund’s rule). • We look at Fe 2+ and Fe 3+ as examples.
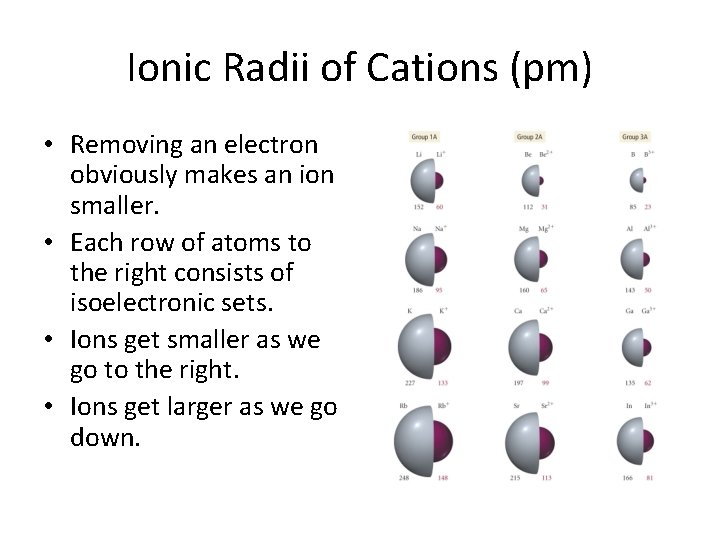
Ionic Radii of Cations (pm) • Removing an electron obviously makes an ion smaller. • Each row of atoms to the right consists of isoelectronic sets. • Ions get smaller as we go to the right. • Ions get larger as we go down.
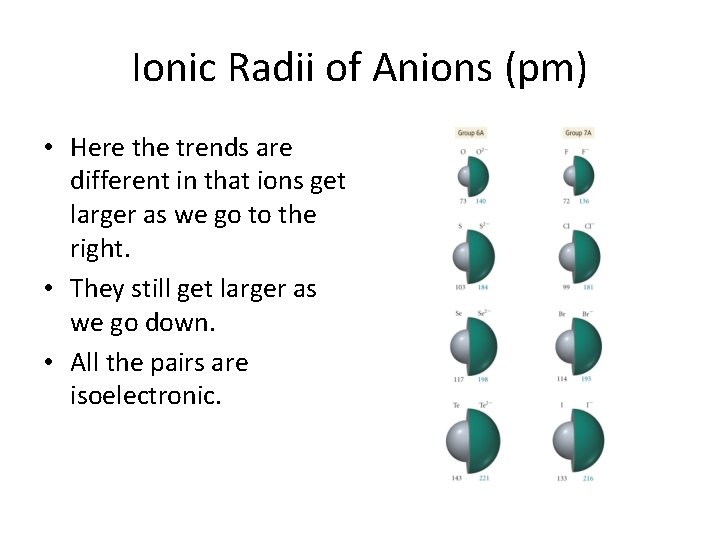
Ionic Radii of Anions (pm) • Here the trends are different in that ions get larger as we go to the right. • They still get larger as we go down. • All the pairs are isoelectronic.

Ionization Energies • Cation formation is generally endothermic. • It takes energy to remove an electron. • Only valence electrons get removed in ordinary chemical reactions. • Core electrons cannot be removed in ordinary reactions! • The next charts show trends in first ionization energies.
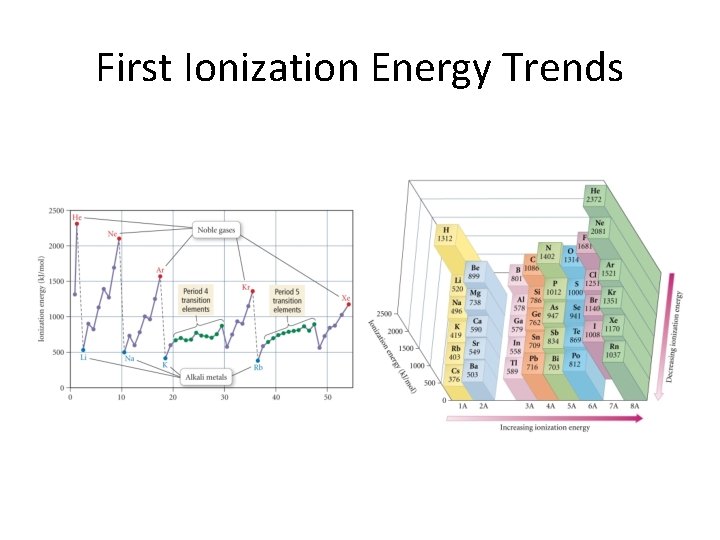
First Ionization Energy Trends

Second and Higher Ionization Energies • Only valence electrons can be removed. • Each successive electron harder to remove since it must now be separated from a cation as opposed to a neutral atom. • Core electrons stay put! • We show these trends in the next two slides.
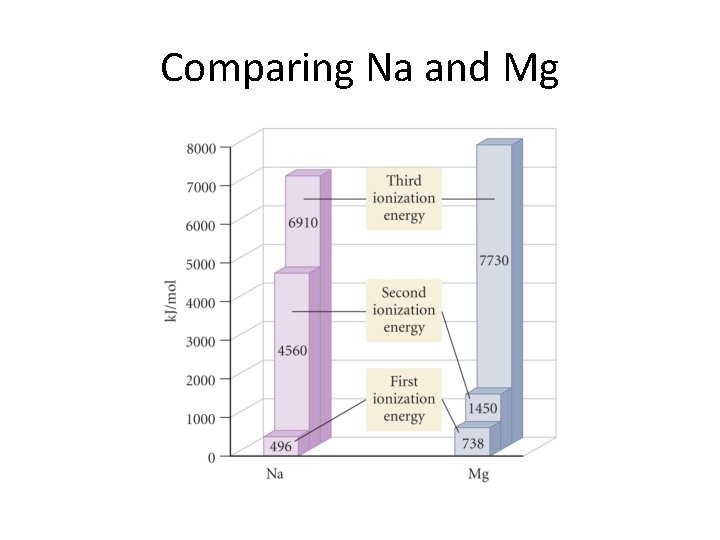
Comparing Na and Mg
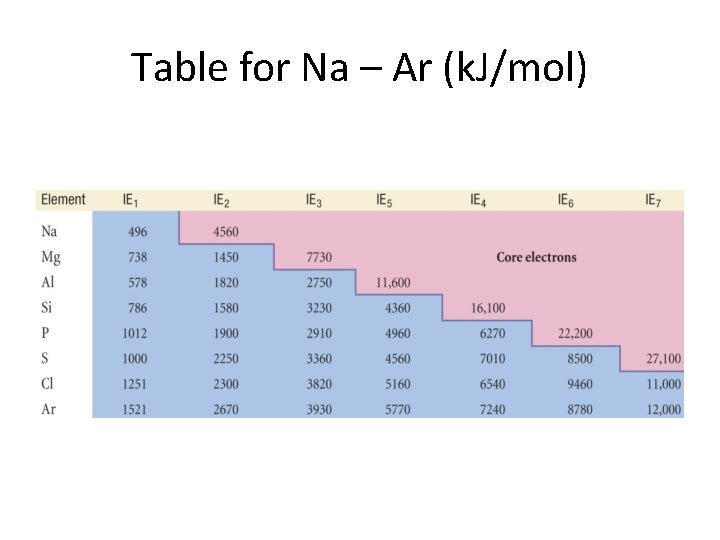
Table for Na – Ar (k. J/mol)

Electron Affinities • This is the energy (usually) given off when a neutral atom receives an electron to become an anion. • Note that this is also (sign change!) the energy needed to convert an electron back into a neutral atom. • The next chart shows some of these for X + e→ X -.
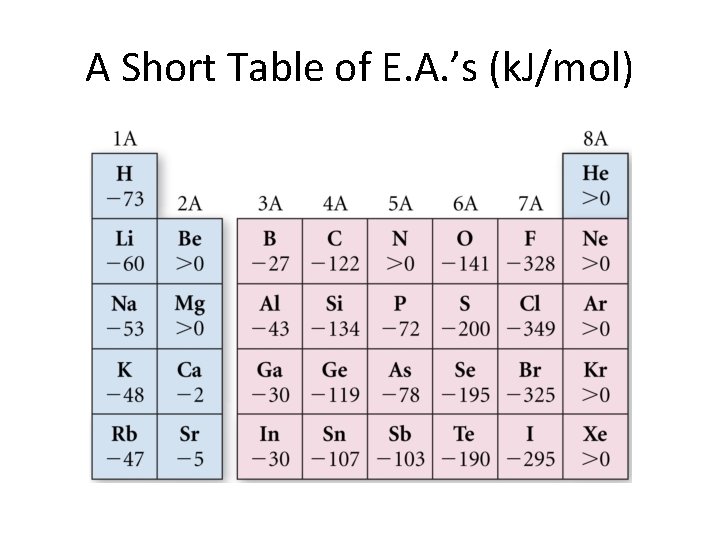
A Short Table of E. A. ’s (k. J/mol)

Three types of elements • • Metals Nonmetals Metalloids We discuss all these while looking at the next slide.
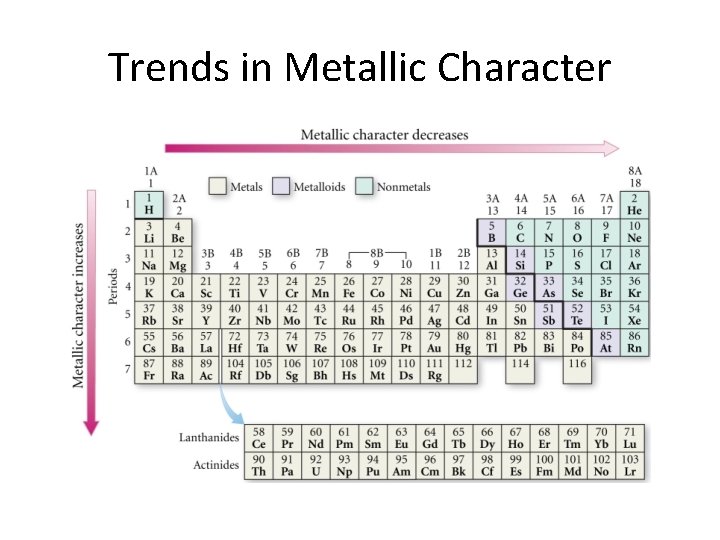
Trends in Metallic Character
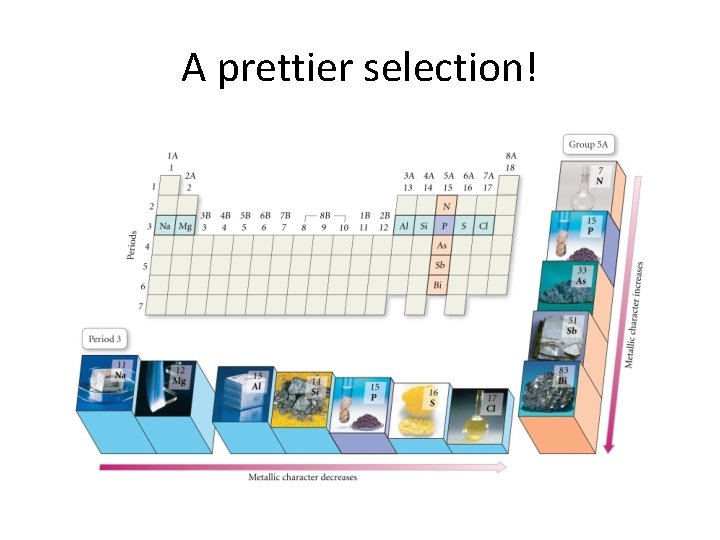
A prettier selection!

At last. . .
- Slides: 81