Periodic Measurement TableFormulas Atoms Physical Molecules Chemical etc
Periodic Measurement Table/Formulas Atoms & Physical, Molecules Chemical, etc Electrons 100 100 100 200 200 200 300 300 300 400 400 400 500 500 500

The measurement 0. 0635 g in scientific notation is:

6. 35 x -2 10 g

Convert 250ºC to Kelvin

523 K

The volume on the graduated cylinder is:

294 m. L

A student lowers a 9. 0 g metal weight into a graduated cylinder. The water level in the graduated cylinder raises from 12. 0 m. L to 14. 7 m. L. Find the density of the metal weight.

3. 33 g/m. L.

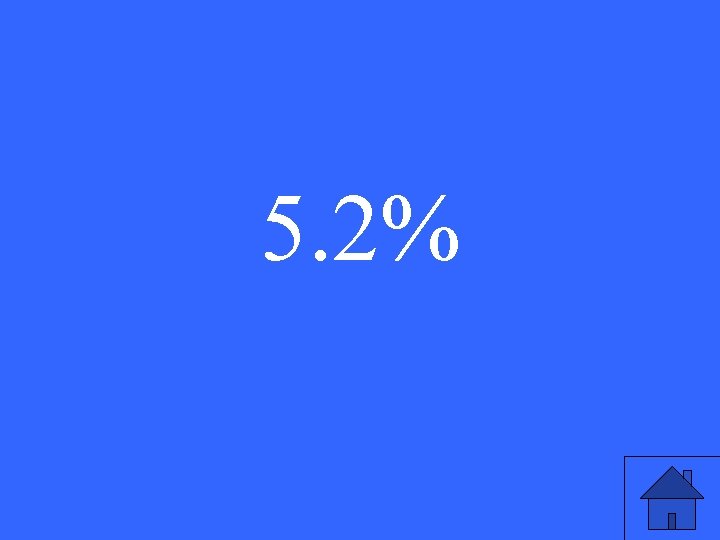
A student does an experiment to find the boiling point of rubbing alcohol. The actual boiling point is 77. 0ºC. He determines the boiling point to be 81°C. Find his percent error.
5. 2%

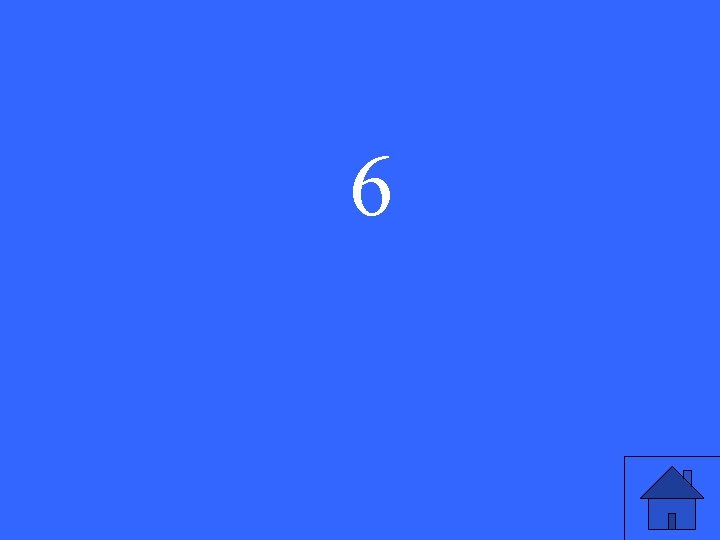
How many occupied energy levels does a barium atom have?
6

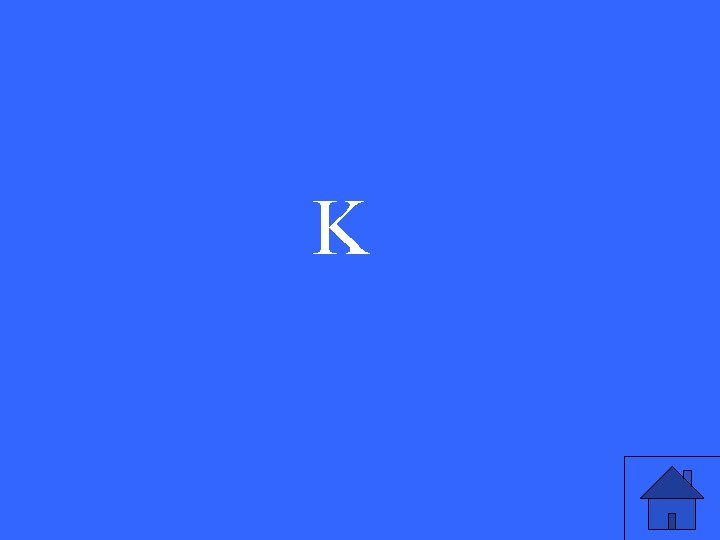
Which element below has the largest radius: Na, K or Cu?
K

• Write the formula for iron (III) sulfate.

• Fe 2(SO 4)3
Arrange the elements below in order of increasing ionization energy S, Cl , Ar

S < Cl < Ar

Name the compound below: H 3 PO 3

• Phosphorous Acid

vocabulary term: quantity which determines the identity of an element
atomic number of protons
1. Draw OCl 2 2. determine its geometry 3. is it polar?
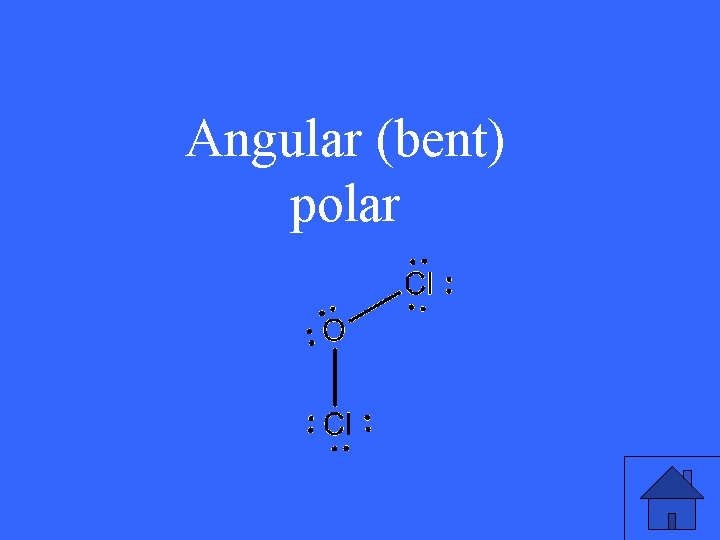
Angular (bent) polar

# of protons, neutrons 52 and electrons in Cr

24 protons 28 neutrons 24 electrons

Find the average atomic mass of the element below: 66 X = 45. 1% 61 X = 52. 4% 62 X = 2. 5%
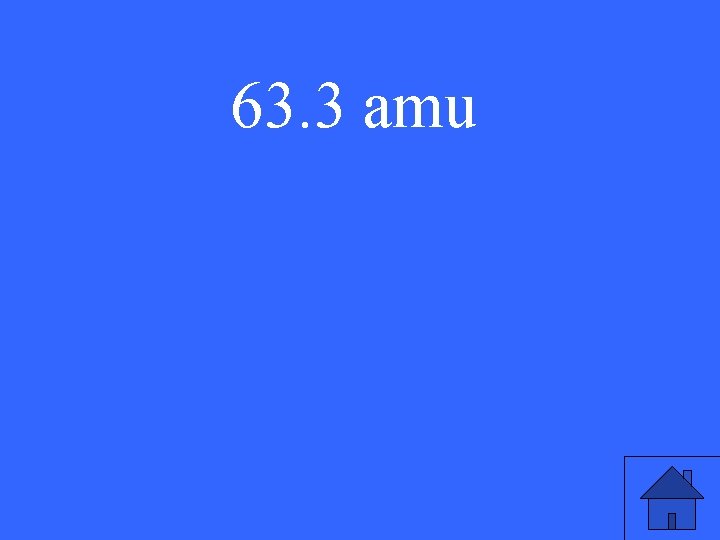
63. 3 amu

Find the number of protons, neutrons and electrons in: 56 Mn 2+

• 25 protons • 31 neutrons • 23 electrons

Physical or Chemical Property? density

physical

Physical or Chemical Change: cooking

chemical

pure substances include:

elements and compounds

Vocabulary: Energy that is stored

Potential energy

Vocabulary: a measure of the average kinetic energy of particles in a sample
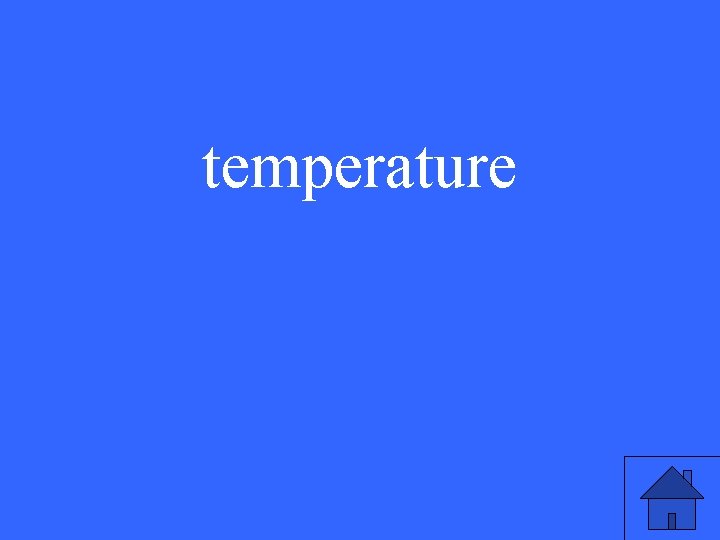
temperature

Vocabulary Term: an atom or group of atoms with an unequal number of protons and electrons

ion

light has properties of:

waves and particles.

abbreviated electron configuration for arsenic
![[Ar] 2 10 4 s 3 d 3 4 p [Ar] 2 10 4 s 3 d 3 4 p](http://slidetodoc.com/presentation_image_h2/0391a9d6b4cc50a93dfbc852d9cb6b4f/image-47.jpg)
[Ar] 2 10 4 s 3 d 3 4 p

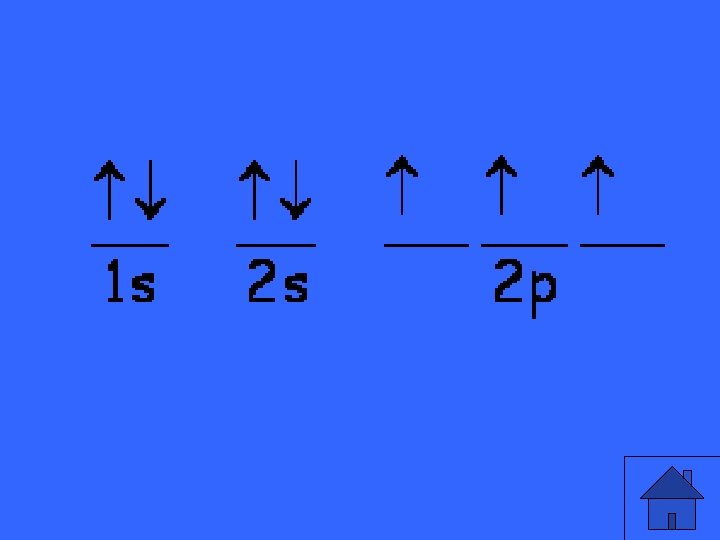
orbital notation for nitrogen:

The wavelength of a wave triples. What will happen to its energy and its frequency? (two separate answers)

The frequency will go down three times. The energy will go down three times.
- Slides: 51