PERCUTANEOUS PULMONARY VALVE REPLACEMENT A NEW PARADIGM Michael




- Slides: 26

PERCUTANEOUS PULMONARY VALVE REPLACEMENT: A NEW PARADIGM Michael C. Slack, M. D.

RELEVANT DISCLOSURES Michael C. Slack, M. D. ● AGA Medical/St. Jude Medical Corporation: Teaching Proctor and Consultant (compensated) ● Siemens AX Division, Forchheim, Germany Pediatric Cardiac Catheterization Laboratories Advisory Board Member (uncompensated)

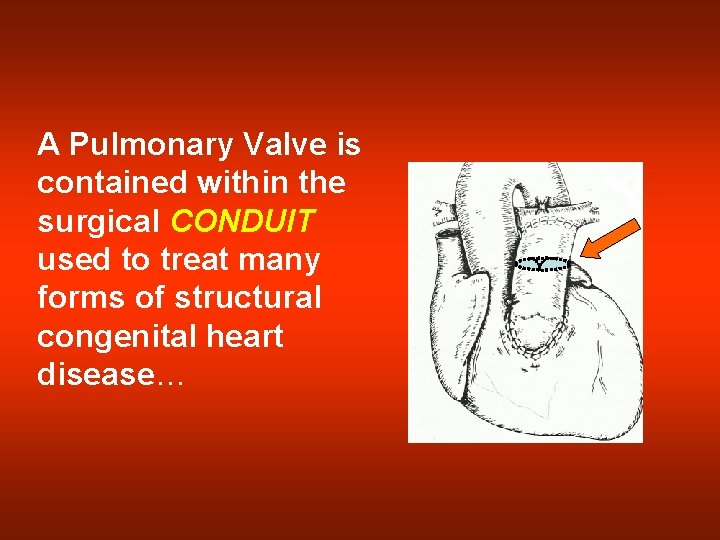
A Pulmonary Valve is contained within the surgical CONDUIT used to treat many forms of structural congenital heart disease…

What are RV to PA CONDUITS? ● Conduits are placed surgically between the right ventricle and the branch pulmonary arteries when no main pulmonary trunk is present or when an alternative path to the pulmonary arteries is required during a complex surgical repair. ● Major types of valved surgical conduits: - human cadaver homografts (pulmonary & aortic) - synthetic material with xenograft valve

Who are the patients that need a CONDUIT that contains a pulmonary valve as part the surgical palliation of their CHD? I. Structural Congenital Heart Defects that require a CONDUIT as part of the repair: ● Pulmonary Atresia ● Tetralogy of Fallot (coronary issues; absent pulmonary valve) ● Truncus Arteriosus ● Double Outlet Right Ventricle (“Rastelli” Repair) ● Transpostion of the Great Arteries (some) II. Other defects: ● Ross Procedure (pulmonary autograft) for AS/AI ● other (endocarditis; early conduit dysfunction, etc. )

All Right Ventricle to Pulmonary Artery CONDUITS Eventually Becomes DYSFUNCTIONAL: Histo-pathologic degrading process occurs & depends on type of conduit: ● pulmonary homografts valve dissolution ● aortic Homografts calcification & valve stenosis ● xenograft conduits combination of valve hardening and internal “rind” formation reducing the lumen diameter of conduit ● variable timeline: 6 mo (early failure) to 8 -10 yrs

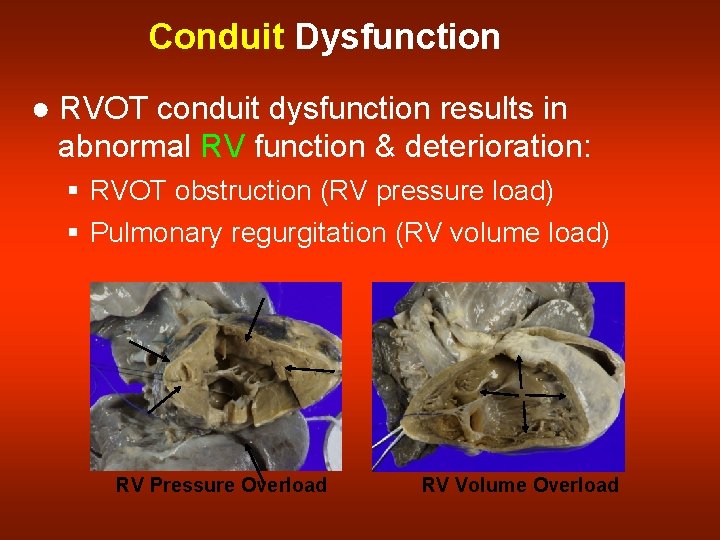
Conduit Dysfunction ● RVOT conduit dysfunction results in abnormal RV function & deterioration: § RVOT obstruction (RV pressure load) § Pulmonary regurgitation (RV volume load) RV Pressure Overload RV Volume Overload

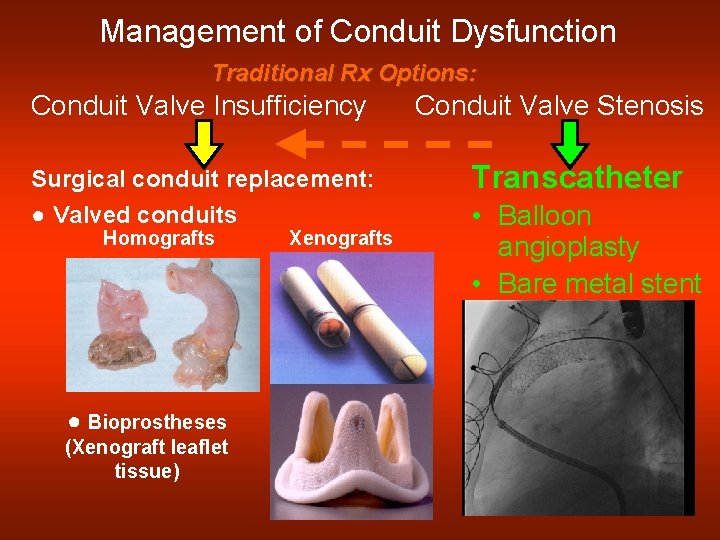
Management of Conduit Dysfunction Traditional Rx Options: Conduit Valve Insufficiency Conduit Valve Stenosis Surgical conduit replacement: Transcatheter ● Valved conduits • Balloon angioplasty • Bare metal stent Homografts ● Bioprostheses (Xenograft leaflet tissue) Xenografts

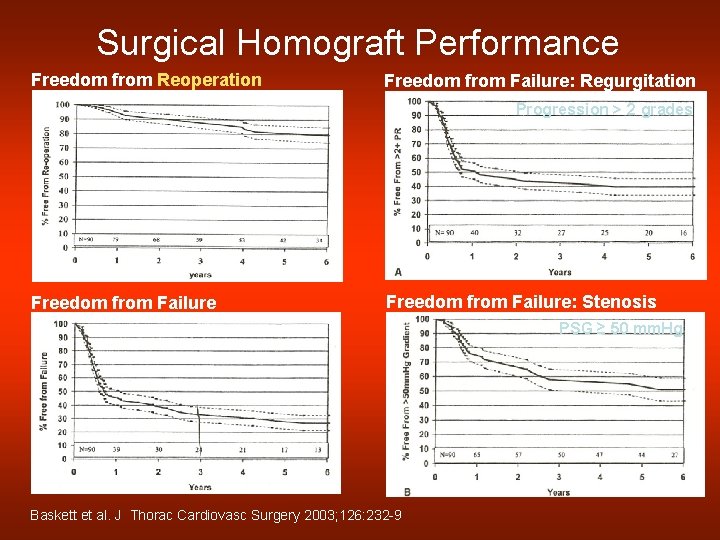
Surgical Homograft Performance Freedom from Reoperation Freedom from Failure: Regurgitation Progression > 2 grades Freedom from Failure: Stenosis PSG ≥ 50 mm. Hg Baskett et al. J Thorac Cardiovasc Surgery 2003; 126: 232 -9

TREATMENT OF CONDUIT VALVE DYSFUNCTION PRE 2010 ALL Required Repeat Open-Heart Surgery: POST- JAN 29, 2010 1 st Percutaneous Pulmonary Valve System Approved in U. S.

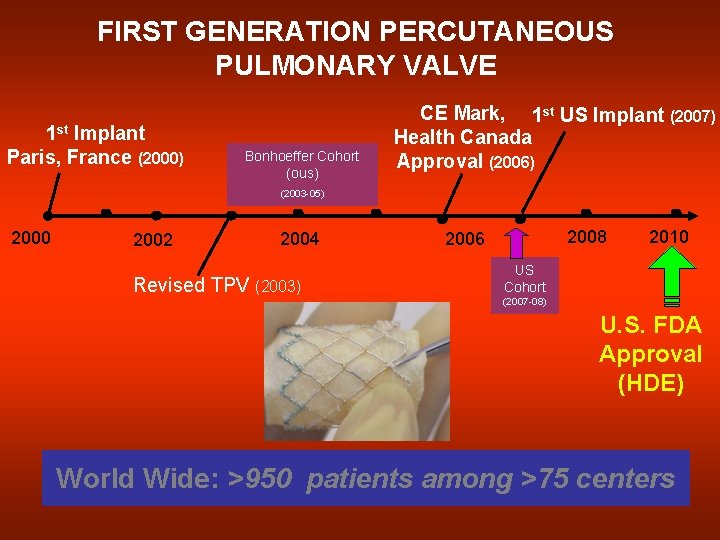
FIRST GENERATION PERCUTANEOUS PULMONARY VALVE 1 st Implant Paris, France (2000) Bonhoeffer Cohort (ous) CE Mark, 1 st US Implant (2007) Health Canada Approval (2006) (2003 -05) 2000 2002 2004 Revised TPV (2003) 2008 2006 2010 US Cohort (2007 -08) U. S. FDA Approval (HDE) World Wide: >950 patients among >75 centers

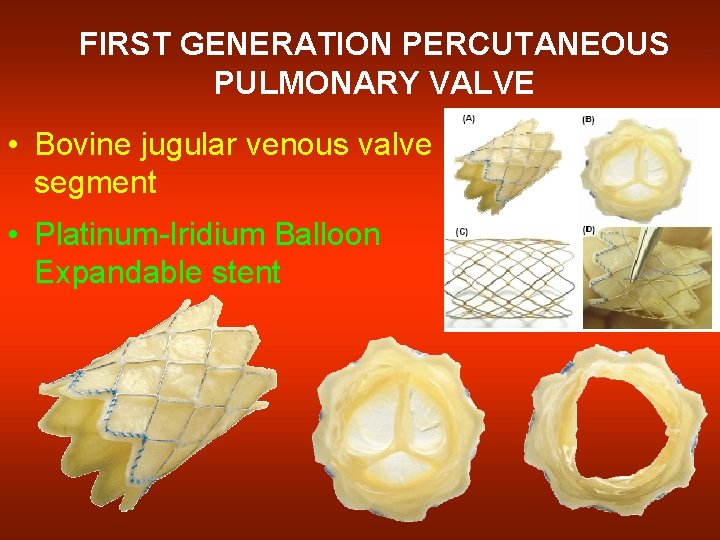
FIRST GENERATION PERCUTANEOUS PULMONARY VALVE • Bovine jugular venous valve segment • Platinum-Iridium Balloon Expandable stent

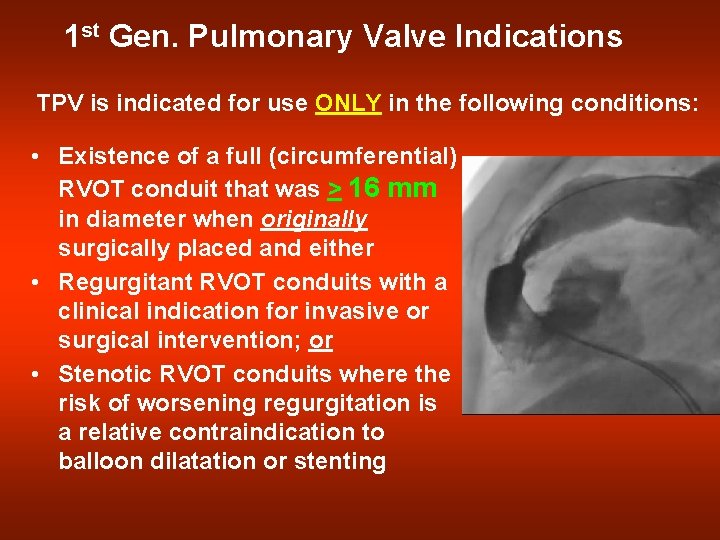
1 st Gen. Pulmonary Valve Indications TPV is indicated for use ONLY in the following conditions: • Existence of a full (circumferential) RVOT conduit that was > 16 mm in diameter when originally surgically placed and either • Regurgitant RVOT conduits with a clinical indication for invasive or surgical intervention; or • Stenotic RVOT conduits where the risk of worsening regurgitation is a relative contraindication to balloon dilatation or stenting

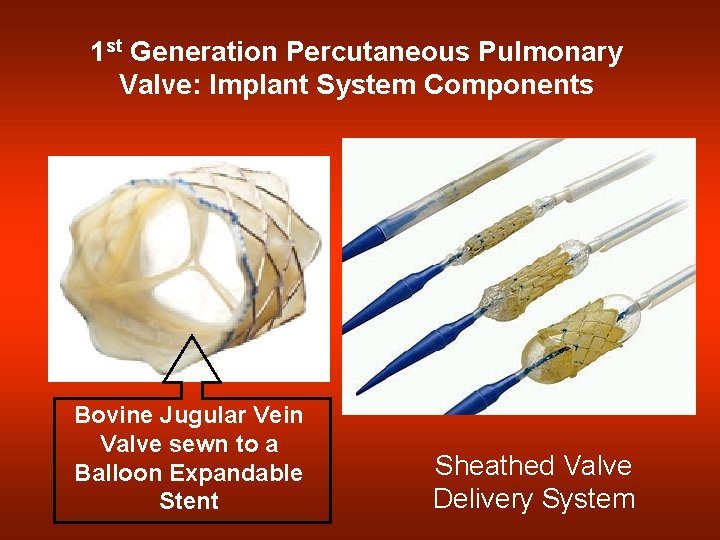
1 st Generation Percutaneous Pulmonary Valve: Implant System Components Bovine Jugular Vein Valve sewn to a Balloon Expandable Stent Sheathed Valve Delivery System

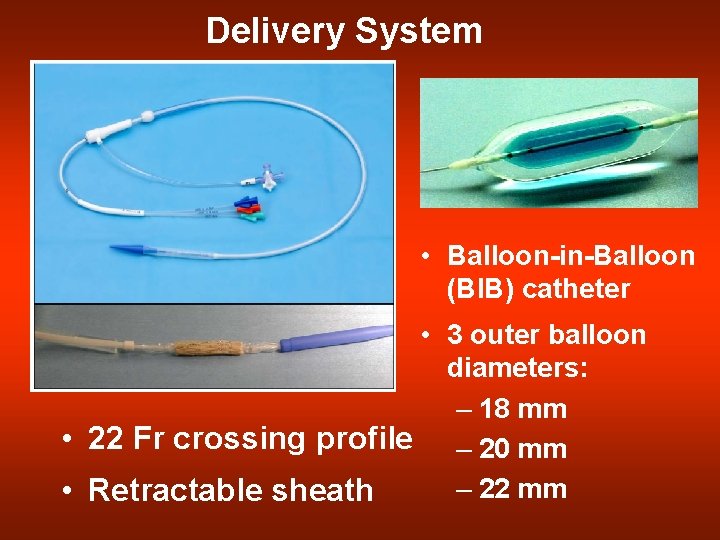
Delivery System • Balloon-in-Balloon (BIB) catheter • 3 outer balloon diameters: – 18 mm • 22 Fr crossing profile – 20 mm – 22 mm • Retractable sheath

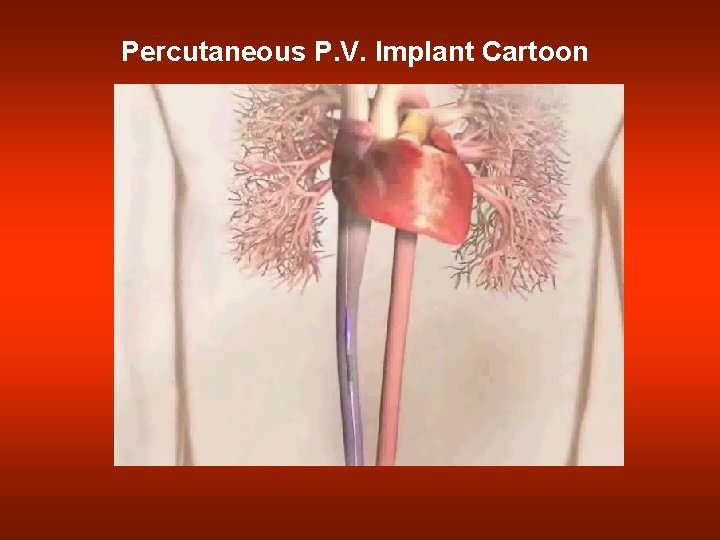
Percutaneous P. V. Implant Cartoon

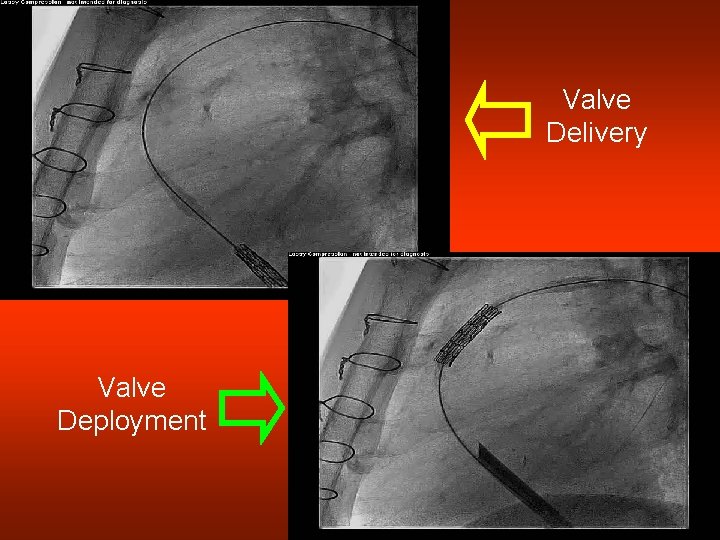
Valve Delivery Valve Deployment

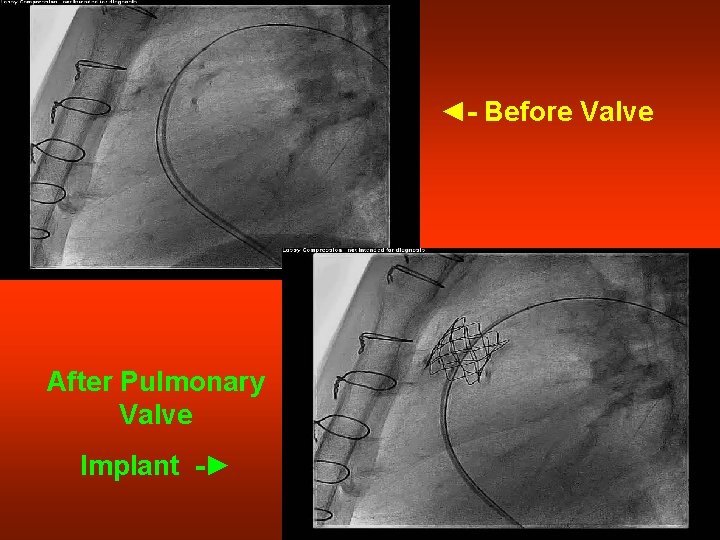
◄- Before Valve After Pulmonary Valve Implant -►

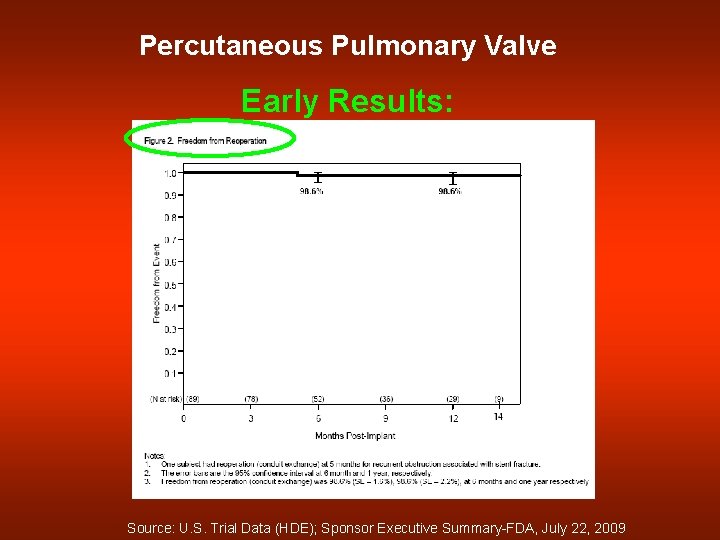
Percutaneous Pulmonary Valve Early Results: Source: U. S. Trial Data (HDE); Sponsor Executive Summary-FDA, July 22, 2009

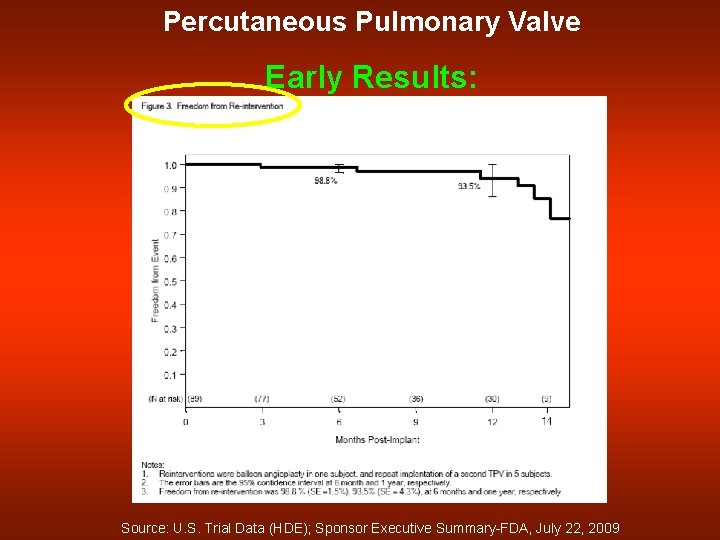
Percutaneous Pulmonary Valve Early Results: Source: U. S. Trial Data (HDE); Sponsor Executive Summary-FDA, July 22, 2009

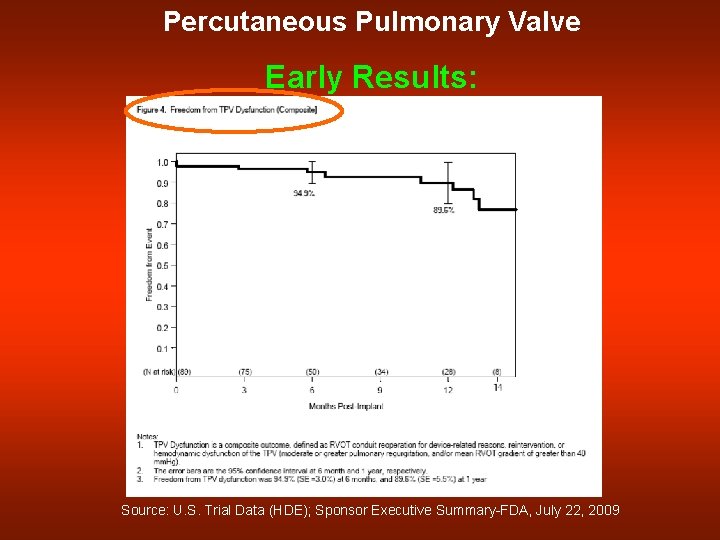
Percutaneous Pulmonary Valve Early Results: Source: U. S. Trial Data (HDE); Sponsor Executive Summary-FDA, July 22, 2009

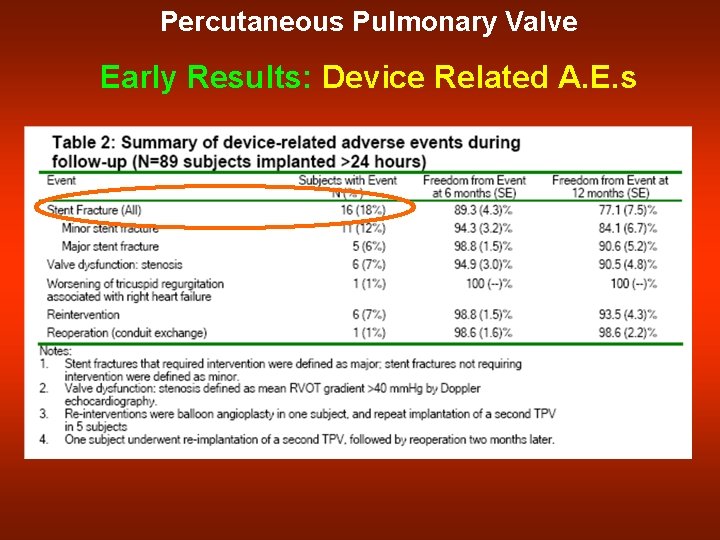
Percutaneous Pulmonary Valve Early Results: Device Related A. E. s

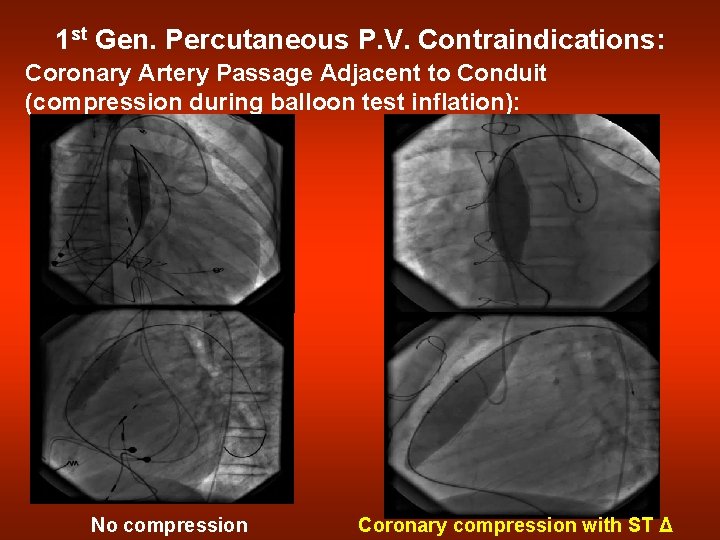
1 st Gen. Percutaneous P. V. Contraindications: Coronary Artery Passage Adjacent to Conduit (compression during balloon test inflation): No compression Coronary compression with ST Δ

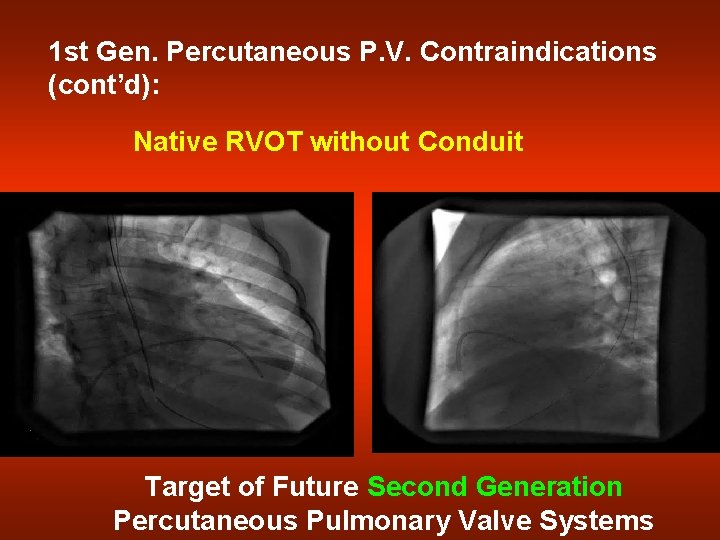
1 st Gen. Percutaneous P. V. Contraindications (cont’d): Native RVOT without Conduit Target of Future Second Generation Percutaneous Pulmonary Valve Systems

Investigational Use Only COMPASSION: COngenital Multicenter Trial of Pulmonic VAlve Regurgitation Studying the SAPIEN Intervent. IONal THV Study began: 5 -2008 Conduits: 16 -24 mm

Thank You!