Percentage Composition Law of Definite Proportions Percent Composition













- Slides: 28

Percentage Composition • Law of Definite Proportions • Percent Composition • Vitamin C application • Percentage Composition from Chemical Formula • Empirical Formula from % Composition • Molecular Formula C 2 H 6 O

Teach. With. Fergy Preview File Please enjoy this preview of your Student Version of the lesson. - Some slides appear blank because they have been removed. -Student versions have portions of the text removed which is given in the teacher version and appear as ______ - Other slides may have. . . on them, this represents writing that has been removed.

Law of Definite Proportions Over 200 years ago, scientists discovered that compounds contained elements in fixed mass proportions. • For example, in 2009 scientists discovered traces of water on the moon. They found this water had the same composition as water on Earth. MH O = 18. 02 g/mol Looking at moles, 1 mol of water then contains 2. 02 g of hydrogen and 16. 00 g of oxygen. 2 _______________________________________________

Law of Definite Proportions con’t. . . We can calculate the mass percent of a compound using its chemical formula. Ex: Find the mass percent of hydrogen in H 2 O mass percent of H = mass of H x 100% mass of H 2 O

THIS SLIDE HAS BEEN REMOVED

Did You Know. . . Humans can’t synthesize vitamin C and therefore must consume it in our diet. However, this wasn’t always possible until 1933 when scientists used percentage composition to synthetically produce vitamin C.

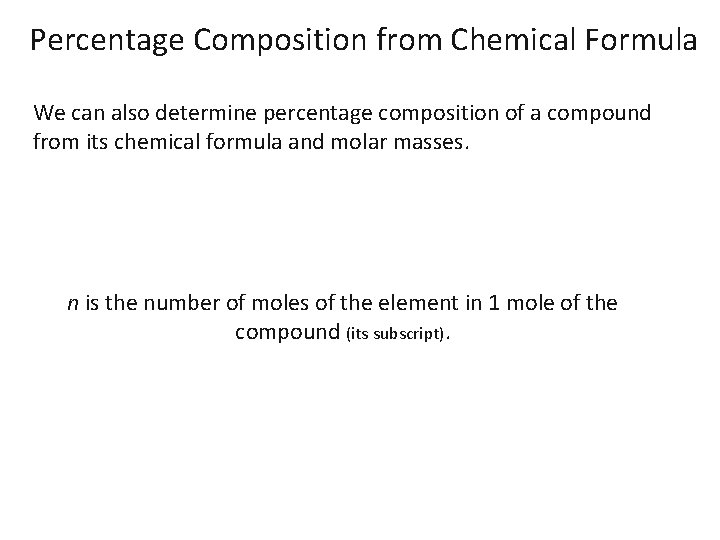
Percentage Composition from Chemical Formula We can also determine percentage composition of a compound from its chemical formula and molar masses. n is the number of moles of the element in 1 mole of the compound (its subscript).

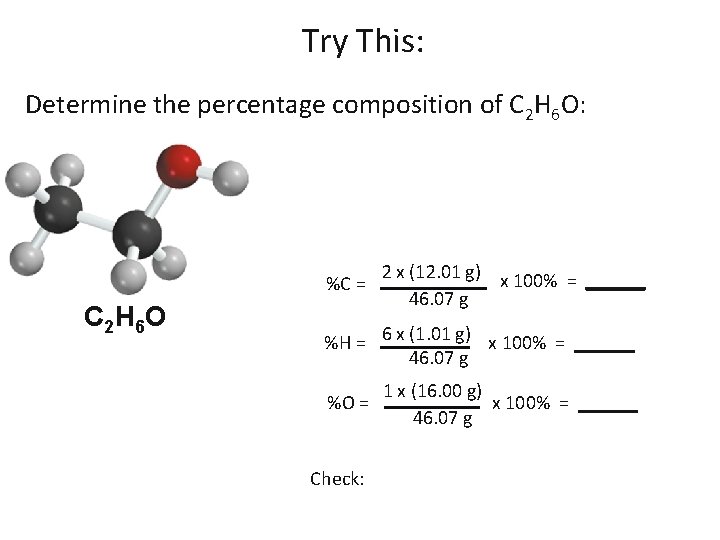
Try This: Determine the percentage composition of C 2 H 6 O: %C = C 2 H 6 O 2 x (12. 01 g) x 100% = ______ 46. 07 g %H = 6 x (1. 01 g) x 100% = ______ 46. 07 g %O = Check: 1 x (16. 00 g) x 100% = ______ 46. 07 g

THIS SLIDE HAS BEEN REMOVED

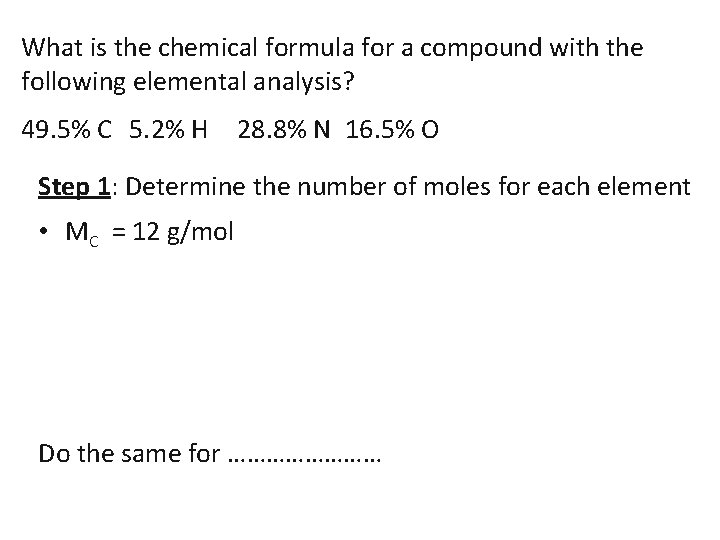
What is the chemical formula for a compound with the following elemental analysis? 49. 5% C 5. 2% H 28. 8% N 16. 5% O Step 1: Determine the number of moles for each element • MC = 12 g/mol Do the same for …………

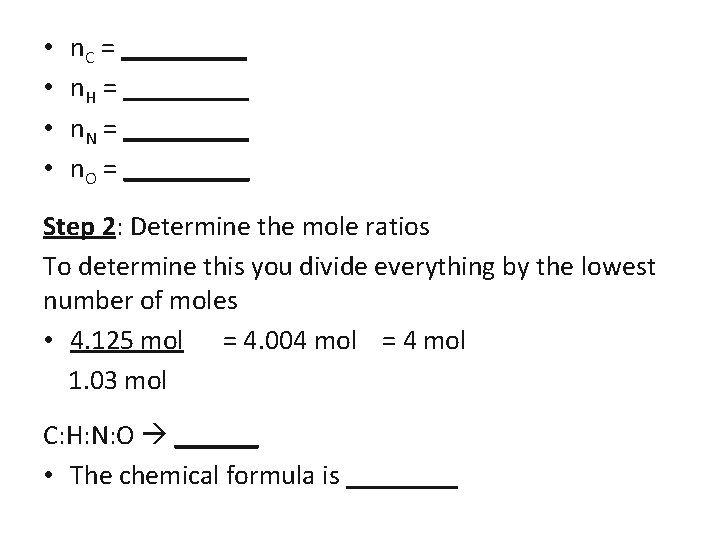
• • n. C = _____ n. H = _____ n. N = _____ n. O = _____ Step 2: Determine the mole ratios To determine this you divide everything by the lowest number of moles • 4. 125 mol = 4. 004 mol = 4 mol 1. 03 mol C: H: N: O ______ • The chemical formula is ____

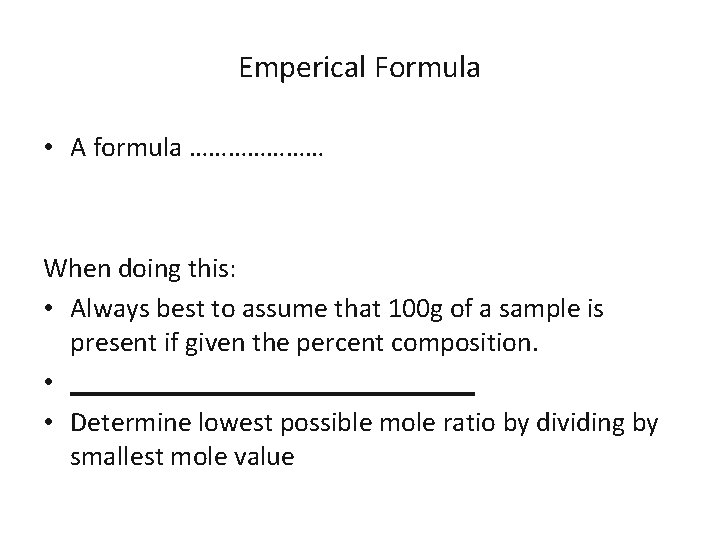
Emperical Formula • A formula ………………… When doing this: • Always best to assume that 100 g of a sample is present if given the percent composition. • _______________ • Determine lowest possible mole ratio by dividing by smallest mole value

THIS SLIDE HAS BEEN REMOVED

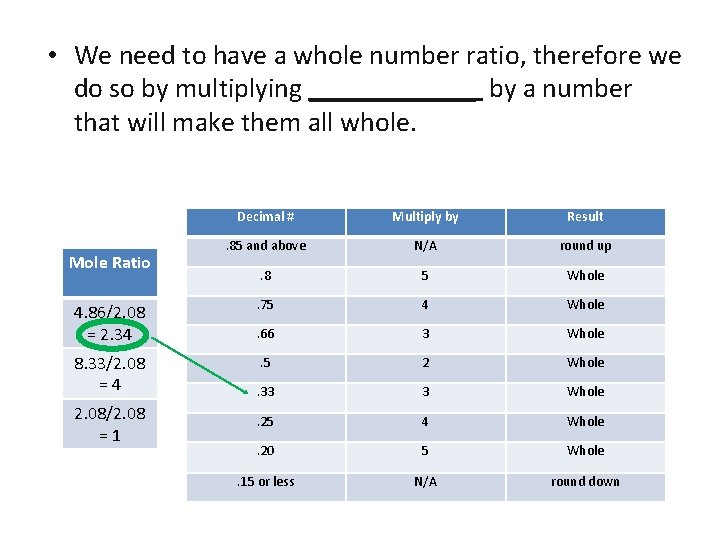
• We need to have a whole number ratio, therefore we do so by multiplying ______ by a number that will make them all whole. Decimal # Multiply by Result . 85 and above N/A round up . 8 5 Whole 4. 86/2. 08 = 2. 34 . 75 4 Whole . 66 3 Whole 8. 33/2. 08 =4 . 5 2 Whole . 33 3 Whole . 25 4 Whole . 20 5 Whole . 15 or less N/A round down Mole Ratio 2. 08/2. 08 =1

Check Your Understanding Calculate the empirical formula for the compound containing: C: 60. 0%, H: 4. 48%, O: 35. 53%

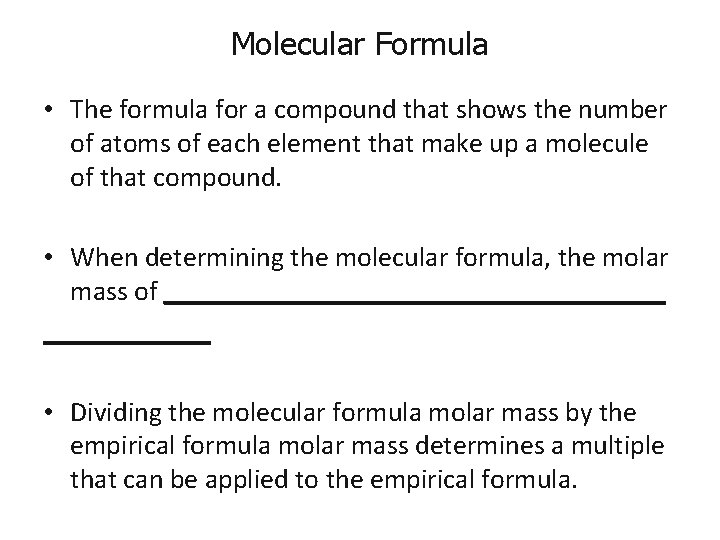
Molecular Formula • The formula for a compound that shows the number of atoms of each element that make up a molecule of that compound. • When determining the molecular formula, the molar mass of __________________ • Dividing the molecular formula molar mass by the empirical formula molar mass determines a multiple that can be applied to the empirical formula.

THIS SLIDE HAS BEEN REMOVED

Check Your Understanding A compound with a molar mass of 180 g/mol is found to be composed of 40% carbon, 6. 65% Hydrogen and 53. 3% Oxygen. Determine the molecular formula.

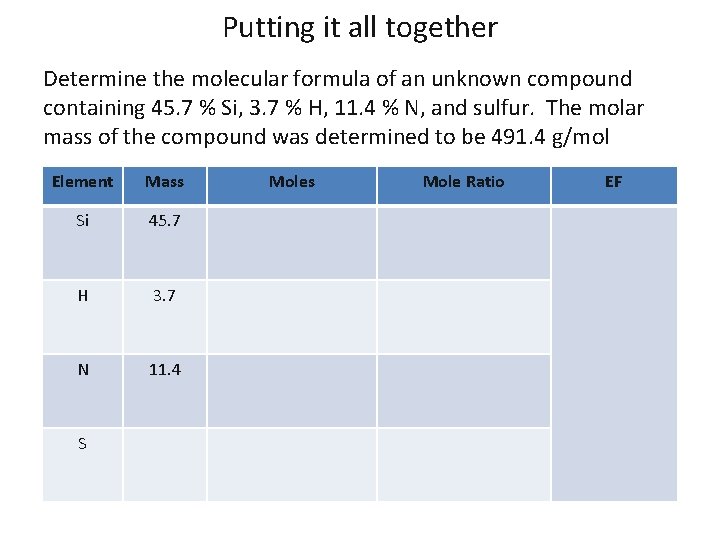
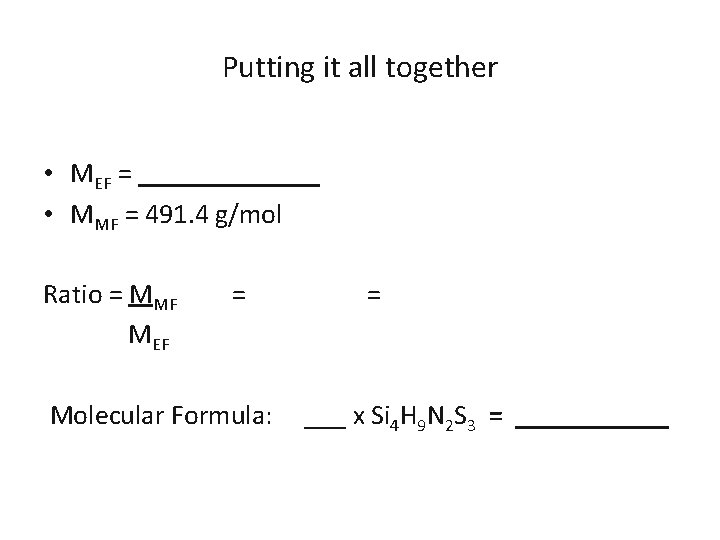
Putting it all together Determine the molecular formula of an unknown compound containing 45. 7 % Si, 3. 7 % H, 11. 4 % N, and sulfur. The molar mass of the compound was determined to be 491. 4 g/mol Element Mass Si 45. 7 H 3. 7 N 11. 4 S Moles Mole Ratio EF

Putting it all together • MEF = _______ • MMF = 491. 4 g/mol Ratio = MMF MEF = Molecular Formula: = ___ x Si 4 H 9 N 2 S 3 = ______

THIS SLIDE HAS BEEN REMOVED

Hydrates

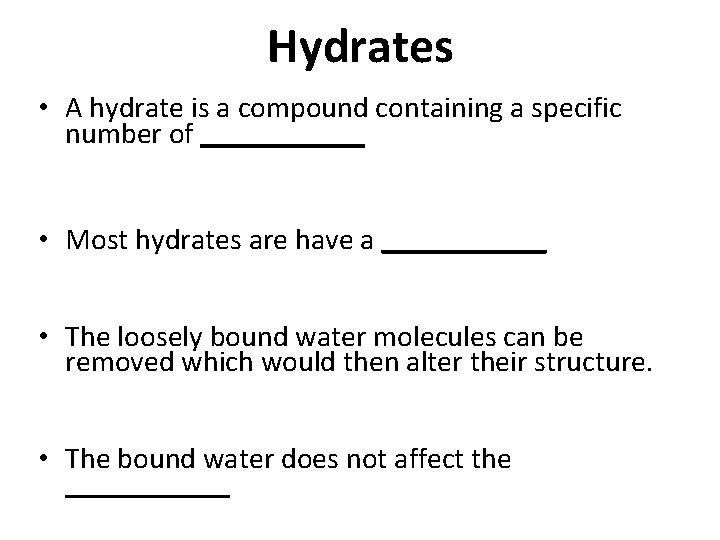
Hydrates • A hydrate is a compound containing a specific number of ______ • Most hydrates are have a ______ • The loosely bound water molecules can be removed which would then alter their structure. • The bound water does not affect the ______

• If you are trying to identify an unknown sample in a lab chances are that no one will supply you with the empirical formula and molar mass. • You are ………………. . • You can do this by _________a known mass (water) by ………… • There are different procedures for different compounds.

THIS SLIDE HAS BEEN REMOVED

Analysis of a Hydrated Salt • We need to know this information because the ______ • The simplest way to determine the amount of water in hydrates is to heat a known mass to release its water. You then determine the mass of the anhydrous salt that ______

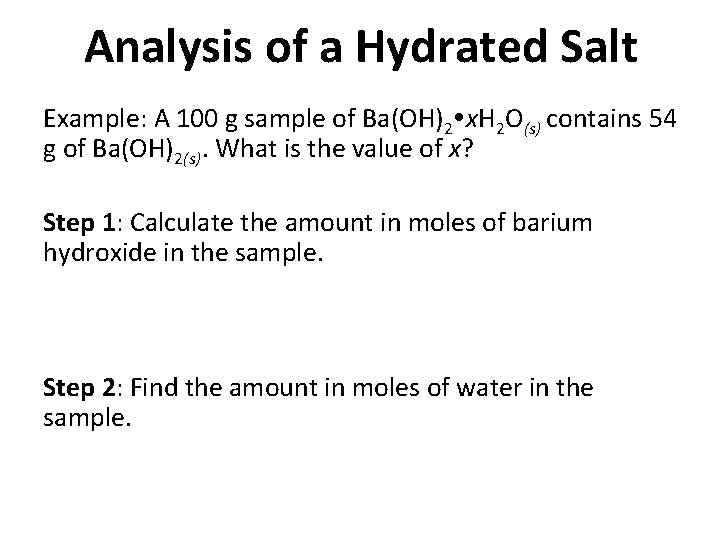
Analysis of a Hydrated Salt Example: A 100 g sample of Ba(OH)2 x. H 2 O(s) contains 54 g of Ba(OH)2(s). What is the value of x? Step 1: Calculate the amount in moles of barium hydroxide in the sample. Step 2: Find the amount in moles of water in the sample.

THIS SLIDE HAS BEEN REMOVED