PERCENTAGE COMPOSITION and EMPIRICAL MOLECULAR FORMULA Percentage Composition

- Slides: 11

PERCENTAGE COMPOSITION and EMPIRICAL & MOLECULAR FORMULA

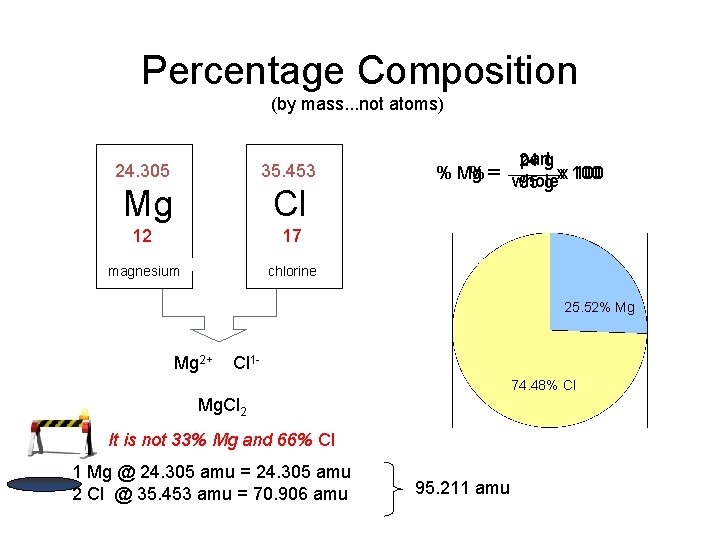
Percentage Composition (by mass. . . not atoms) 24. 305 35. 453 Mg Cl 12 17 magnesium chlorine partg 24 % Mg % == whole 100 95 g xx 100 25. 52% Mg Mg 2+ Cl 174. 48% Cl Mg. Cl 2 It is not 33% Mg and 66% Cl 1 Mg @ 24. 305 amu = 24. 305 amu 2 Cl @ 35. 453 amu = 70. 906 amu 95. 211 amu

Empirical and Molecular Formulas A pure compound always consists of the same elements combined in the same proportions by weight. Therefore, we can express molecular composition as PERCENT BY WEIGHT Ethanol, C 2 H 6 O 52. 13% C 13. 15% H 34. 72% O

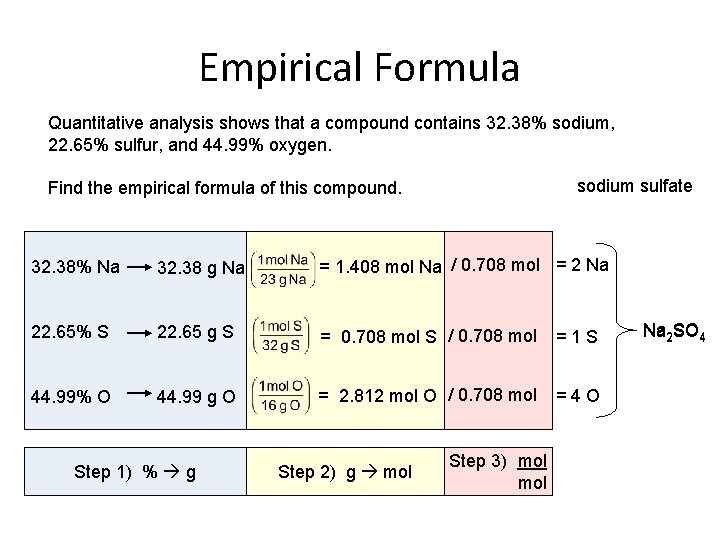
Empirical Formula Quantitative analysis shows that a compound contains 32. 38% sodium, 22. 65% sulfur, and 44. 99% oxygen. sodium sulfate Find the empirical formula of this compound. 32. 38% Na 32. 38 g Na = 1. 408 mol Na / 0. 708 mol = 2 Na 22. 65% S 22. 65 g S = 0. 708 mol S / 0. 708 mol =1 S 44. 99% O 44. 99 g O = 2. 812 mol O / 0. 708 mol =4 O Step 1) % g Step 2) g mol Step 3) mol Na 2 SO 4

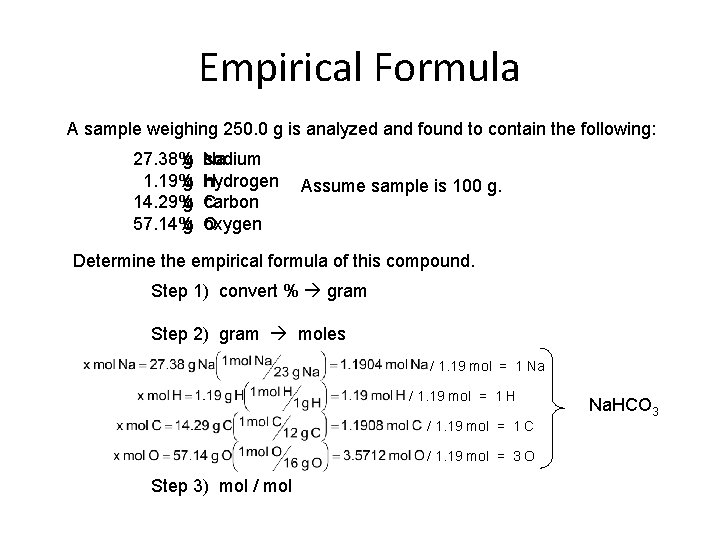
Empirical Formula A sample weighing 250. 0 g is analyzed and found to contain the following: 27. 38 g 27. 38% 1. 19 g 14. 29% 14. 29 g 57. 14% 57. 14 g Na sodium H hydrogen C carbon O oxygen Assume sample is 100 g. Determine the empirical formula of this compound. Step 1) convert % gram Step 2) gram moles / 1. 19 mol = 1 Na / 1. 19 mol = 1 H / 1. 19 mol = 1 C / 1. 19 mol = 3 O Step 3) mol / mol Na. HCO 3

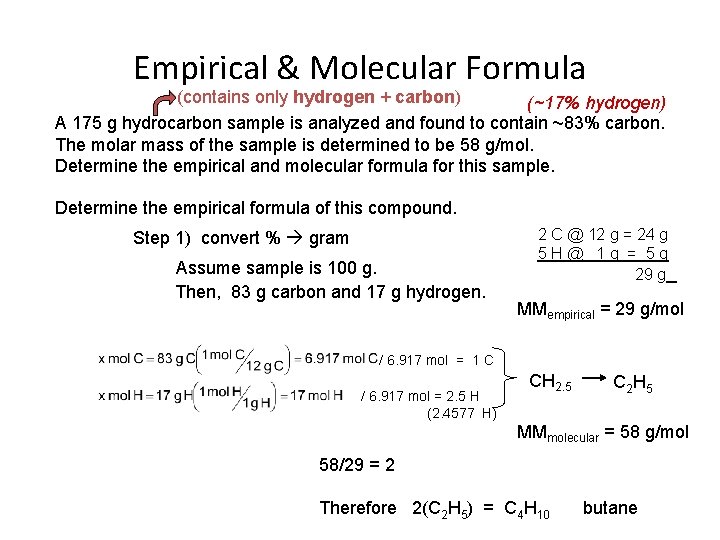
Empirical & Molecular Formula (contains only hydrogen + carbon) (~17% hydrogen) A 175 g hydrocarbon sample is analyzed and found to contain ~83% carbon. The molar mass of the sample is determined to be 58 g/mol. Determine the empirical and molecular formula for this sample. Determine the empirical formula of this compound. Step 1) convert % gram Assume sample is 100 g. Then, 83 g carbon and 17 g hydrogen. Step 2) gram moles 2 C @ 12 g = 24 g 5 H@ 1 g = 5 g 29 g MMempirical = 29 g/mol / 6. 917 mol = 1 C / 6. 917 mol = 2. 5 H (2. 4577 H) CH 2. 5 C 2 H 5 MMmolecular = 58 g/mol Step 3) mol / mol 58/29 = 2 Therefore 2(C 2 H 5) = C 4 H 10 butane

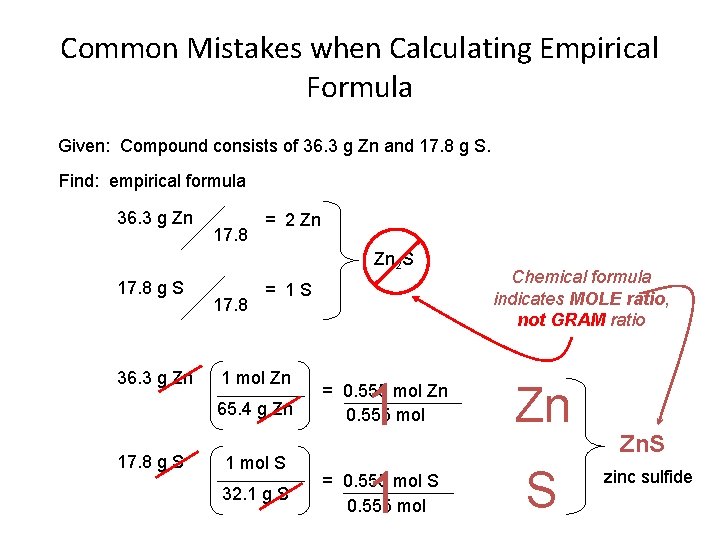
Common Mistakes when Calculating Empirical Formula Given: Compound consists of 36. 3 g Zn and 17. 8 g S. Find: empirical formula 36. 3 g Zn 17. 8 = 2 Zn Zn 2 S 17. 8 g S 36. 3 g Zn 17. 8 = 1 S 1 mol Zn 65. 4 g Zn 17. 8 g S 1 mol S 32. 1 g S 1 1 = 0. 555 mol Zn 0. 555 mol = 0. 555 mol S 0. 555 mol Chemical formula indicates MOLE ratio, not GRAM ratio Zn S Zn. S zinc sulfide

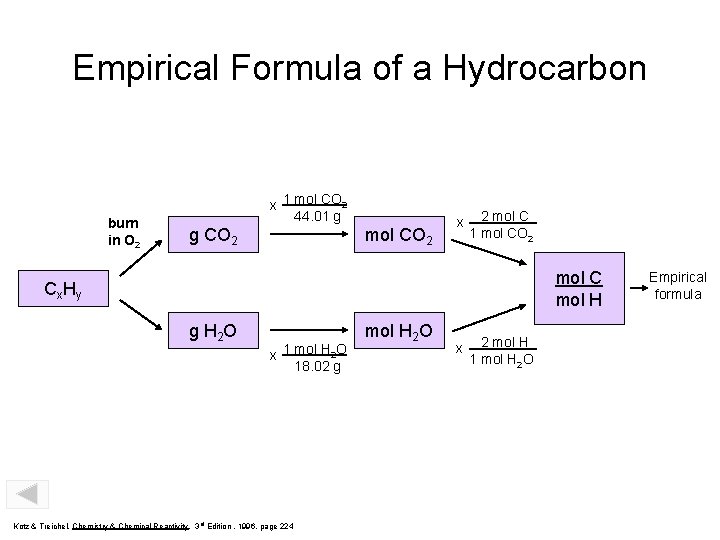
Empirical Formula of a Hydrocarbon burn in O 2 x 1 mol CO 2 44. 01 g g CO 2 mol CO 2 x 2 mol C 1 mol CO 2 mol C mol H Cx Hy g H 2 O x 1 mol H 2 O 18. 02 g Kotz & Treichel, Chemistry & Chemical Reactivity, 3 rd Edition , 1996, page 224 mol H 2 O x 2 mol H 1 mol H 2 O Empirical formula

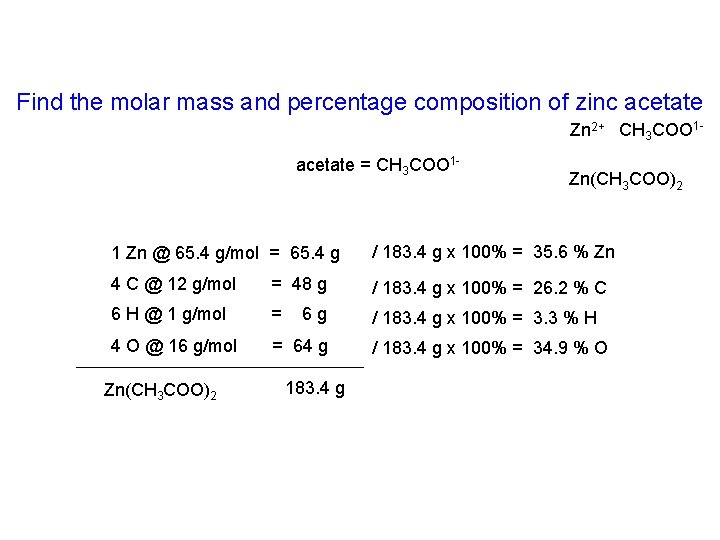
Find the molar mass and percentage composition of zinc acetate Zn 2+ CH 3 COO 1 acetate = CH 3 COO 1 - Zn(CH 3 COO)2 1 Zn @ 65. 4 g/mol = 65. 4 g / 183. 4 g x 100% = 35. 6 % Zn 4 C @ 12 g/mol = 48 g / 183. 4 g x 100% = 26. 2 % C 6 H @ 1 g/mol = / 183. 4 g x 100% = 3. 3 % H 4 O @ 16 g/mol = 64 g Zn(CH 3 COO)2 6 g 183. 4 g / 183. 4 g x 100% = 34. 9 % O

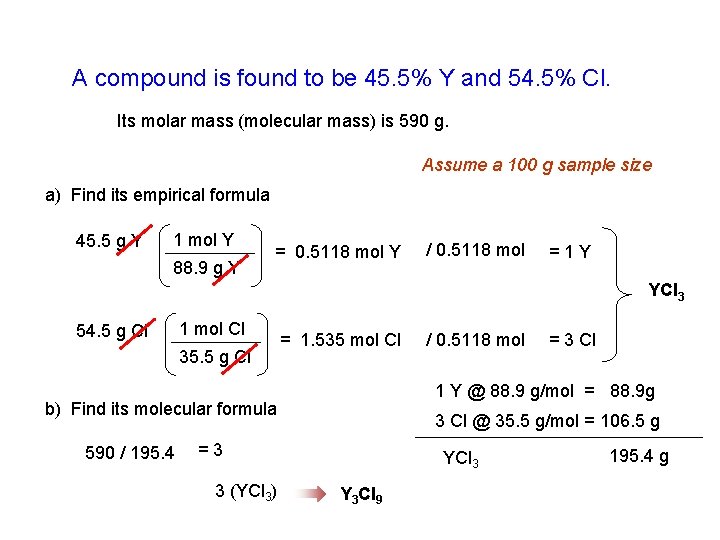
A compound is found to be 45. 5% Y and 54. 5% Cl. Its molar mass (molecular mass) is 590 g. Assume a 100 g sample size a) Find its empirical formula 45. 5 g Y 1 mol Y 88. 9 g Y = 0. 5118 mol Y / 0. 5118 mol =1 Y YCl 3 54. 5 g Cl 1 mol Cl 35. 5 g Cl = 1. 535 mol Cl 3 Cl @ 35. 5 g/mol = 106. 5 g =3 3 (YCl 3) = 3 Cl 1 Y @ 88. 9 g/mol = 88. 9 g b) Find its molecular formula 590 / 195. 4 / 0. 5118 mol YCl 3 Y 3 Cl 9 195. 4 g

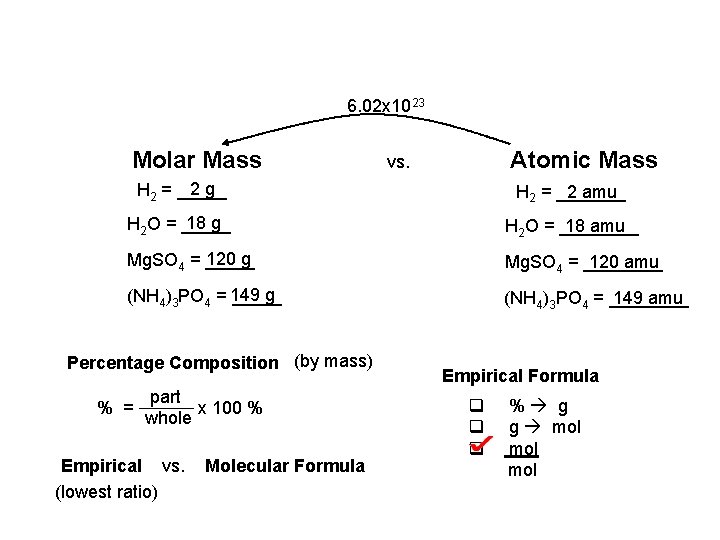
6. 02 x 1023 Molar Mass Atomic Mass vs. 2 g H 2 = _______ 2 amu 18 g H 2 O = ________ 18 amu 120 g Mg. SO 4 = ________ 120 amu g (NH 4)3 PO 4 = 149 _____ (NH 4)3 PO 4 = ____ 149 amu Percentage Composition (by mass) % = part x 100 % whole Empirical vs. (lowest ratio) Molecular Formula Empirical Formula q q q % g g mol mol