Percent Composition Percent Composition by Mass If you
Percent Composition
+ + Percent Composition by Mass – If you want to know what percentage of a compounds’ mass is contributed to by each element present.
• Example: If you have a box containing 100 golf balls and 100 ping pong balls, which type of ball contributes the most to the MASS of the box? – The same principle applies to finding the % composition of a compound. Different elements have different masses and this must be taken into consideration.
Why chemists use it? • To figure out which compounds are the best sources of an element. • What if I wanted to use potassium oxide as a source of oxygen, it would be helpful to know the % of oxygen is in the compound.
How to find the percent composition of a compound: 1. Write a correct formula for the compound. 2. Find the molar mass of the compound. (calculated from the periodic table) 3. Divide the total atomic mass of EACH ELEMENT by the molar mass of the compound. 4. Multiply by 100 to convert your results to a percent. 5. Express your answer to TWO decimal places and don’t forget the % sign
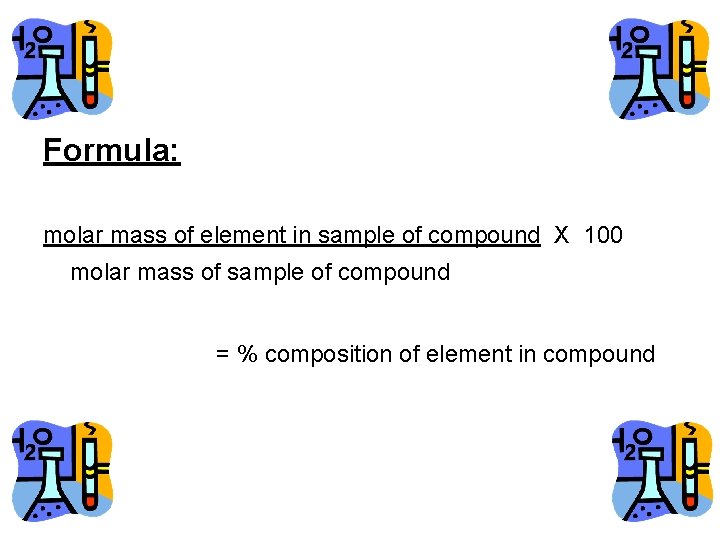
Formula: molar mass of element in sample of compound X 100 molar mass of sample of compound = % composition of element in compound

Example 1: Find the percent composition of oxygen in potassium oxide.
Example 2: Find the percent composition of copper (I) sulfate.
- Slides: 8