Percent Composition of Compounds Empirical Formulas An Introduction













- Slides: 22

Percent Composition of Compounds & Empirical Formulas An Introduction to Analytical Chemistry Packet #21

Introduction • When utilizing a cellular phone, a limited amount of space is provided. • Certain apps require more space than others. • The amount of space used by a particular app can be defined as the percent of memory used by that app.

Percent Composition • Percent composition is defined as a measure of how much of a given substance has been mixed with another substance. • The relative amounts of the element in a compound • The percent mass of each element in a compound.

Percent Composition Equation • ((Mass of element present in 1 mole of compound) / (Molar Mass of compound)) * 100

Example #1 • Carvone is a substance that occurs in two forms, both of which have the same molecular formula (C 10 H 14 O) and molar mass. One type of carvone gives caraway seeds their characteristic smell; the other is responsible for the smell of spearmint oil. Compute the mass percent of each element in carvone.

Example #2 • Propane C 3 H 8, the fuel commonly used in gas grills, is one of the compounds obtained from petroleum. Calculate the percent composition of propane. {Hint: -What is the mass percent of each element in propane? }

Percent Concentration • When a solution is prepared in chemistry, the chemist is usually interested in the solution’s strength or concentration. • Concentration is a measure of the quantity of solute dissolved to a given amount of solvent or solution. Solution Solute Solvent

Percent Concentration • A more precise way of expressing concentration is to specify the quantity of solute that is dissolved in one hundred parts of the solution. • This expression is called percent concentration. Solution Solute Solvent

Percent Concentration Equation Mass Percent Concentration • ((Mass of solute) / (Mass of solution)) * 100

Example #3 • A student dissolves 25. 0 grams of glucose in 475 grams of water. What is the mass percent concentration of the glucose solution?

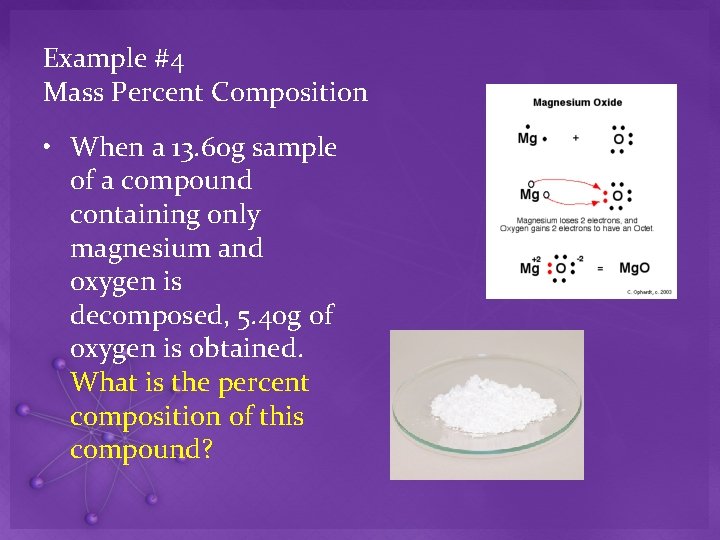
Example #4 Mass Percent Composition • When a 13. 60 g sample of a compound containing only magnesium and oxygen is decomposed, 5. 40 g of oxygen is obtained. What is the percent composition of this compound?

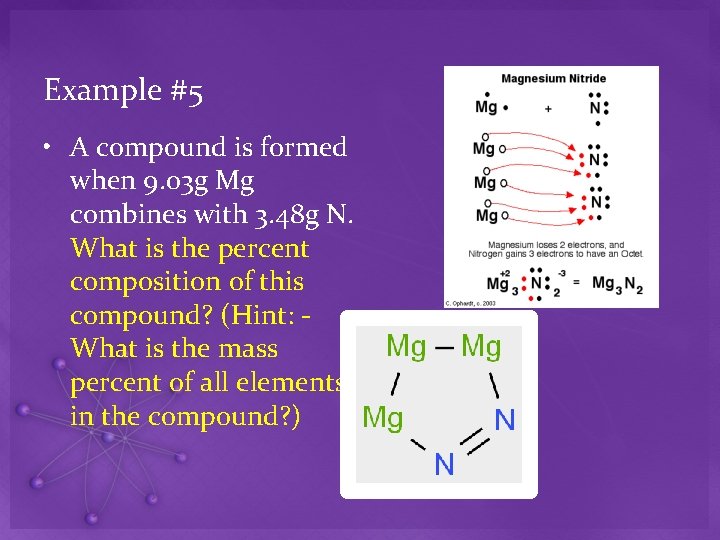
Example #5 • A compound is formed when 9. 03 g Mg combines with 3. 48 g N. What is the percent composition of this compound? (Hint: What is the mass percent of all elements in the compound? )

Example #6 • When a 14. 2 g sample of mercury (II) oxide is decomposed into its elements by heating, 13. 2 g of Hg is obtained. What is the percent composition of this compound?

Example #7 • Calculate the percent nitrogen in these common fertilizers. • NH 3 • NH 4 NO 3

EMPIRICAL FORMULA

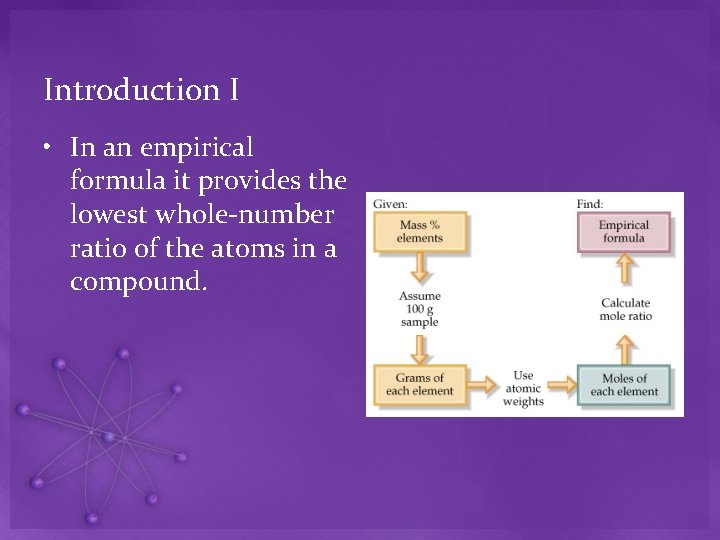
Introduction I • In an empirical formula it provides the lowest whole-number ratio of the atoms in a compound.

Introduction II • Remember, in the empirical formula, the elements appear in smallest whole-number ratios. • In the molecular formula H 2 O, the ratio of hydrogen to oxygen cannot be reduced further—hence it is also an empirical formula. • H 2 n. On • The empirical formula shows the smallest whole-number ratio of the atoms in the compound while the molecular formula tells the actual number of each kind of atom present in the compound.

Example #1 • A compound is analyzed and found to contain 25. 9% nitrogen and 74. 1% oxygen. What is the empirical formula of the compound?

Example #2 • Calculate the molecular formula of a compound whose molar mass is 60. 0 g/mol and empirical formula is Cn. H 4 n. Nn.

Example #3 • Find the molecular formula of ethylene glycol , which is used as antifreeze. The molar mass is 62 g/mol and the empirical formula is Cn. H 3 n. On.

Example #4 • Which pair of molecules has the same empirical formula? • C 2 H 4 O 2; C 6 H 12 O 6 • Na. Cr. O 4; Na 2 Cr 2 O 7

Review