PERCENT COMPOSITION Lab 17 What is the percent
PERCENT COMPOSITION Lab # 17 What is the percent composition of US pennies? OR What’s on the inside?
INTRODUCTION Pennies are made of zinc with a thin layer of copper. Their composition has changed throughout the years. The cost of making pennies has risen as the value of copper has increased.

INTRODUCTION The composition was pure copper from 1793 to 1837. From 1857, the cent was 88%copper and 12%nickel. The cent was 95% copper, and five% tin and zinc from 1864 to 1962. In 1943, the coin's composition was changed to zinc coated steel due to the critical use of copper for the war effort.

PURPOSE The purpose of this lab will be to determine the percent composition of the penny. We will determine the percentage of zinc and copper found in modern pennies (after 1982). We will react our pennies with concentrated Hydrochloric acid. It will react with the zinc but not the copper.

PROCEDURE 1) Weigh the penny on the balance and record it in your data table. 2. 50 g

PROCEDURE 2) Using the metal cutting shears place 2 -4 small cuts in the penny be sure to see the silver color of the inside.

PROCEDURE 3) Place the penny into a jar.

PROCEDURE 4) Measure out 5 ml of the concentrated hydrochloric acid. BE SURE TO HAVE YOUR GOGGLES ON!!!!!

PROCEDURE 5) Pour the acid into the jar covering the penny. Look for bubbles to start forming. Set aside for a day.
PROCEDURE (next day) 6) Pour the remaining acid into the waste Jar with the funnel. Rinse out your jar.

PROCEDURE (next day) 7) Remove the penny from the jar using tweezers. Rinse the penny in the base solution. Pat dry. BASE

PROCEDURE 8) Weigh the penny on the balance and record it in your data table. Mass of the penny after the reaction. 0. 075 g
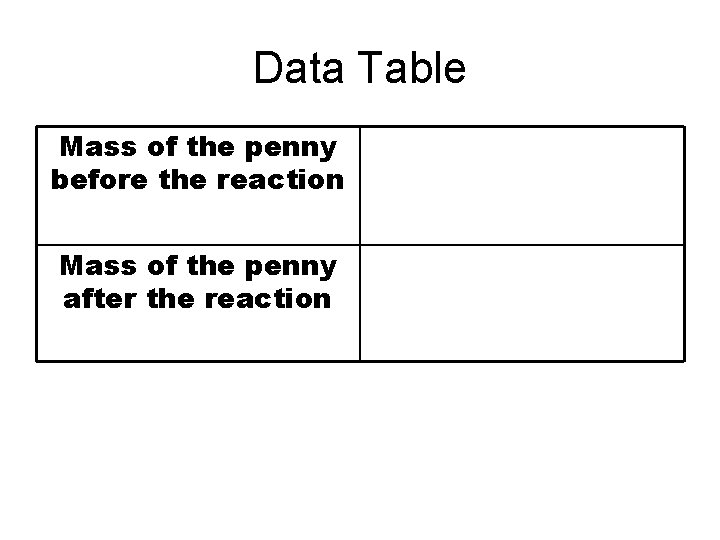
Data Table Mass of the penny before the reaction Mass of the penny after the reaction
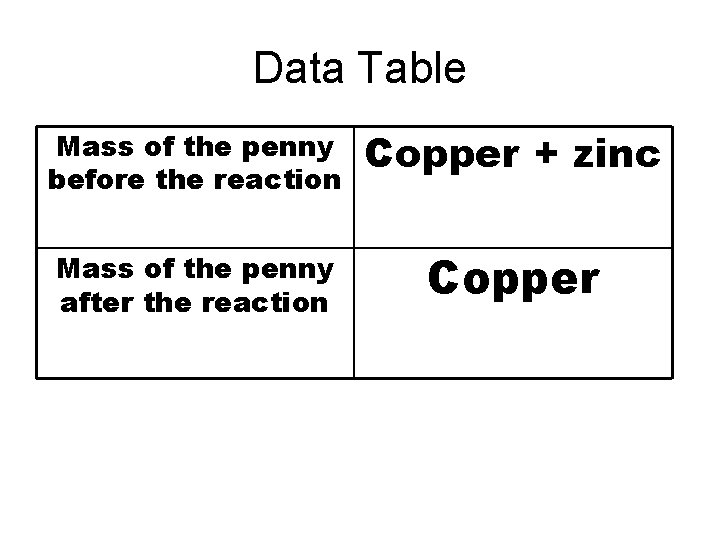
Data Table Mass of the penny before the reaction Copper + zinc Mass of the penny after the reaction Copper
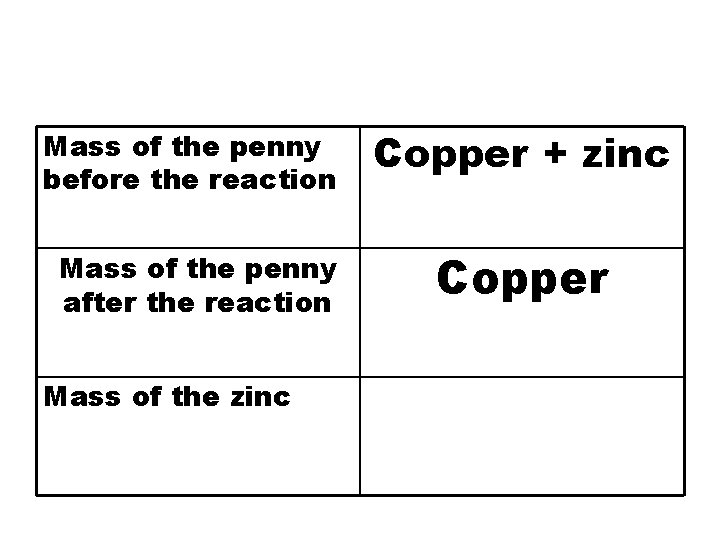
Mass of the penny before the reaction Copper + zinc Mass of the penny after the reaction Copper Mass of the zinc
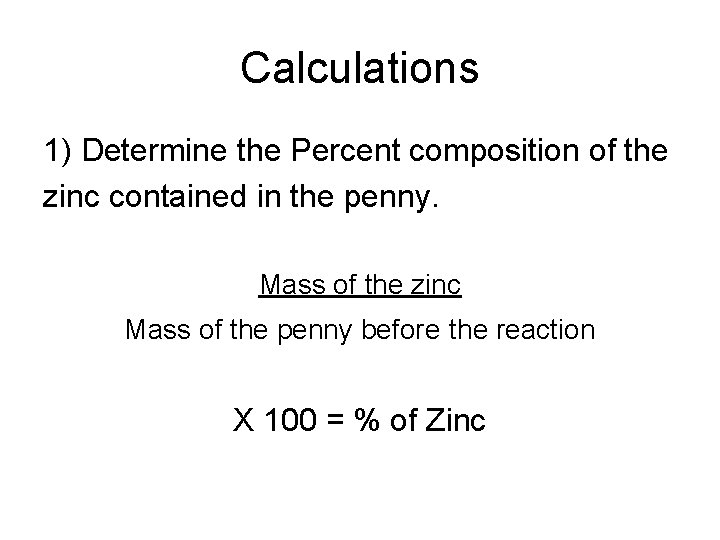
Calculations 1) Determine the Percent composition of the zinc contained in the penny. Mass of the zinc Mass of the penny before the reaction X 100 = % of Zinc
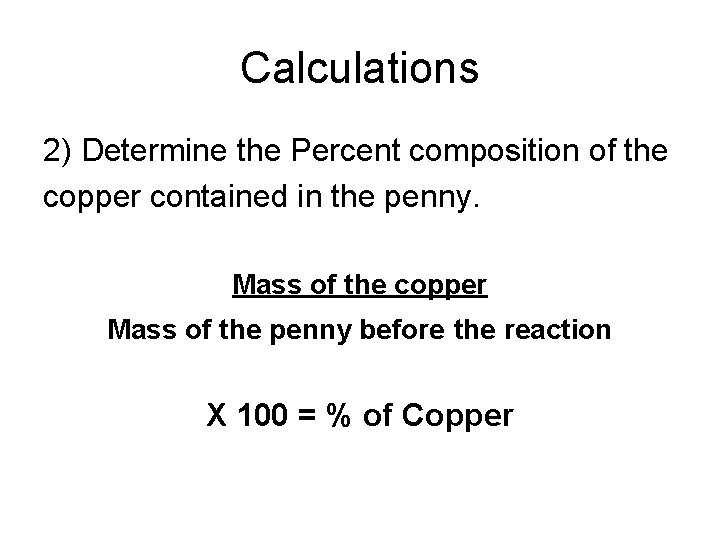
Calculations 2) Determine the Percent composition of the copper contained in the penny. Mass of the copper Mass of the penny before the reaction X 100 = % of Copper

Calculations 3) Write a word equation to describe the chemical reaction that occurred.
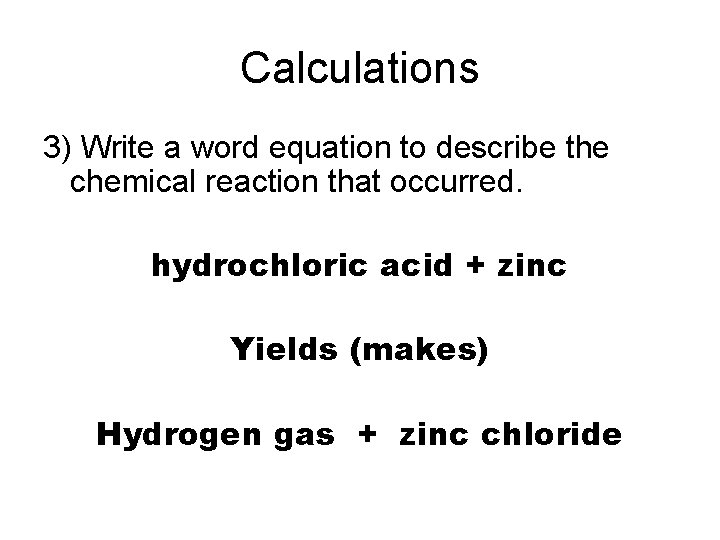
Calculations 3) Write a word equation to describe the chemical reaction that occurred. hydrochloric acid + zinc Yields (makes) Hydrogen gas + zinc chloride
- Slides: 19