Percent Composition Empirical Formulas Molecular Formulas Percent Composition

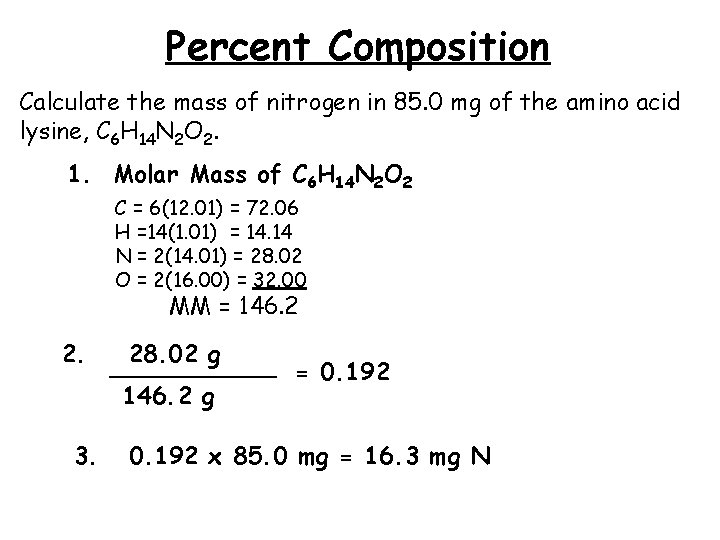

- Slides: 30

Percent Composition, Empirical Formulas, Molecular Formulas

Percent Composition • Percent Composition – the percentage by mass of each element in a compound Part _______ Percent = x 100% Whole So… Percent composition Mass of element in 1 mol of a compound or = __________ x 100% Mass of 1 molecule

Percent Composition Example: What is the percent composition of Potassium Permanganate (KMn. O 4)? Molar Mass of KMn. O 4 K = 1(39. 1) = 39. 1 Mn = 1(54. 9) = 54. 9 O = 4(16. 0) = 64. 0 MM = 158 g

Percent Composition Example: What is the percent composition of Potassium Permanganate (KMn. O 4)? Molar Mass of KMn. O 4 % K 39. 1 g K 158 g = 158 g x 100 = 24. 7 % 54. 9 g Mn x 100 = 34. 8 % % Mn 158 g K= 1(39. 10) = 39. 1 Mn = 1(54. 94) = 54. 9 O = 4(16. 00) = 64. 0 MM = 158 64. 0 g O x 100 = 40. 5 % % O 158 g

Percent Composition Determine the percentage composition of sodium carbonate (Na 2 CO 3)? Molar Mass Na = 2(23. 00) = 46. 0 C = 1(12. 01) = 12. 0 O = 3(16. 00) = 48. 0 MM= 106 g Percent Composition 46. 0 g % Na =106 g 12. 0 g % C = 106 g 48. 0 g % O = 106 g x 100% = 43. 4 % x 100% = 11. 3 % x 100% = 45. 3 %

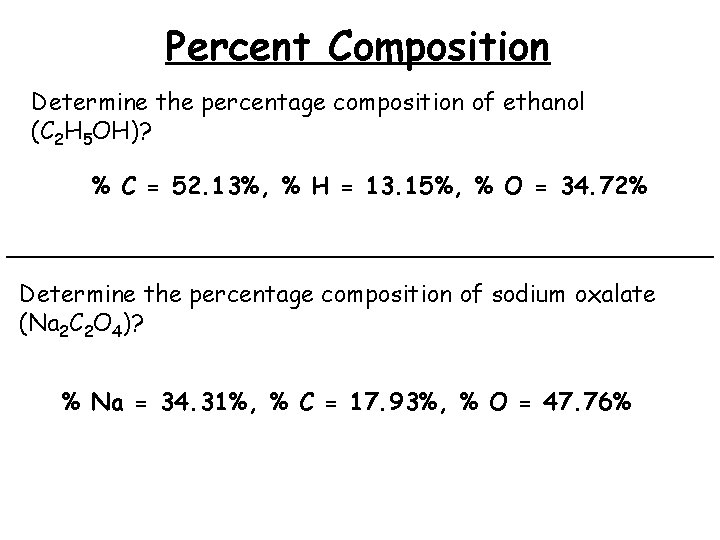
Percent Composition Determine the percentage composition of ethanol (C 2 H 5 OH)? % C = 52. 13%, % H = 13. 15%, % O = 34. 72% ________________________ Determine the percentage composition of sodium oxalate (Na 2 C 2 O 4)? % Na = 34. 31%, % C = 17. 93%, % O = 47. 76%

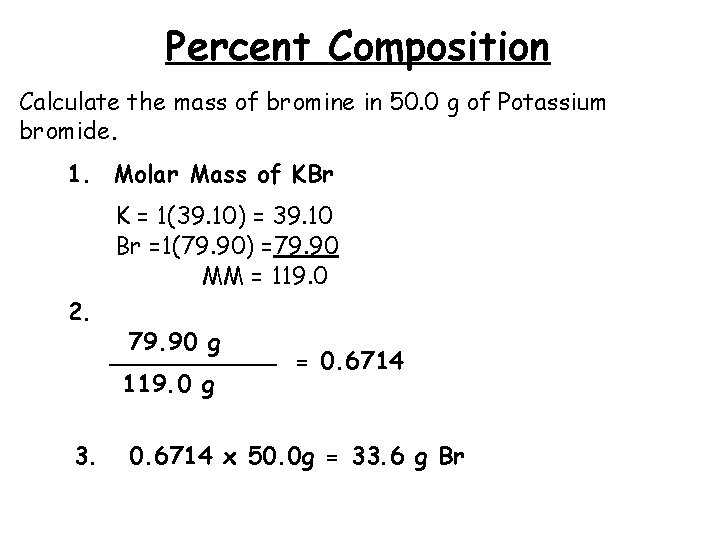
Percent Composition Calculate the mass of bromine in 50. 0 g of Potassium bromide. 1. Molar Mass of KBr K = 1(39. 10) = 39. 10 Br =1(79. 90) =79. 90 MM = 119. 0 2. 3. 79. 90 g ______ = 0. 6714 119. 0 g 0. 6714 x 50. 0 g = 33. 6 g Br
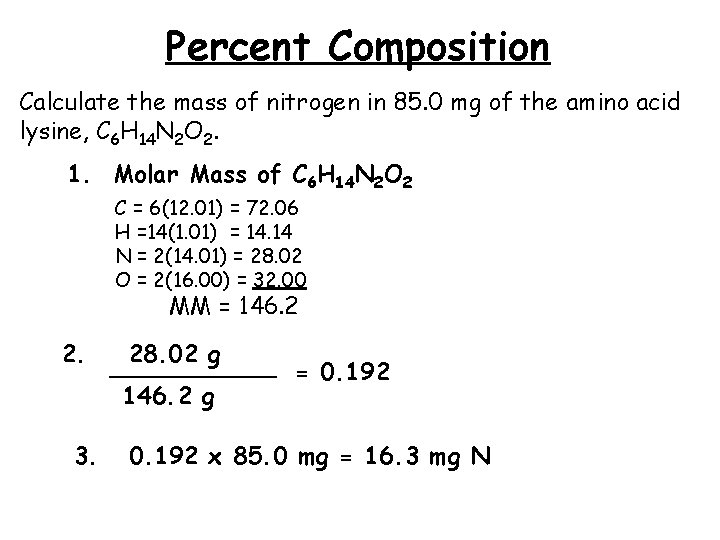
Percent Composition Calculate the mass of nitrogen in 85. 0 mg of the amino acid lysine, C 6 H 14 N 2 O 2. 1. Molar Mass of C 6 H 14 N 2 O 2 C = 6(12. 01) = 72. 06 H =14(1. 01) = 14. 14 N = 2(14. 01) = 28. 02 O = 2(16. 00) = 32. 00 MM = 146. 2 2. 3. 28. 02 g ______ = 0. 192 146. 2 g 0. 192 x 85. 0 mg = 16. 3 mg N

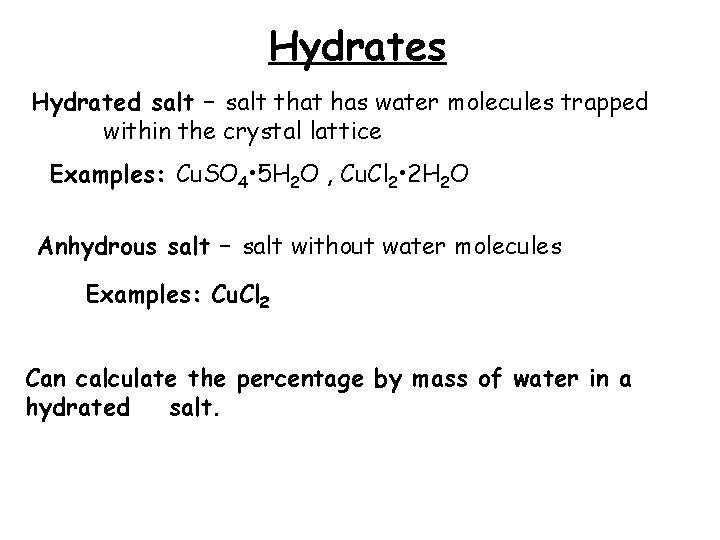
Hydrates Hydrated salt – salt that has water molecules trapped within the crystal lattice Examples: Cu. SO 4 • 5 H 2 O , Cu. Cl 2 • 2 H 2 O Anhydrous salt – salt without water molecules Examples: Cu. Cl 2 Can calculate the percentage by mass of water in a hydrated salt.

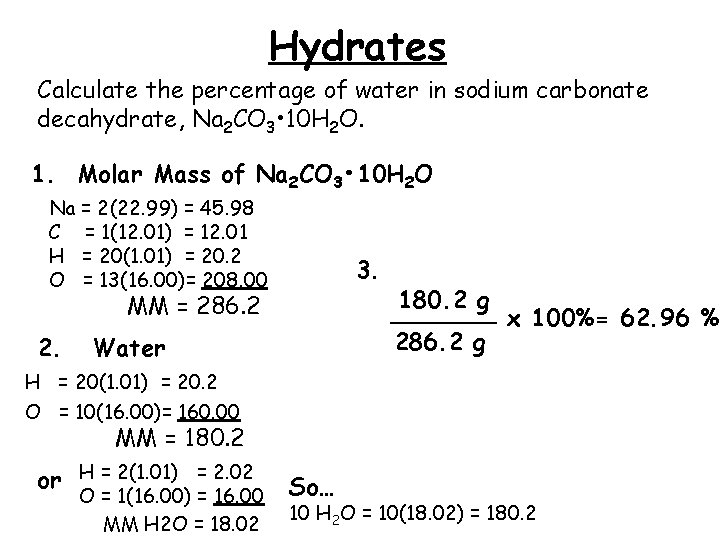
Hydrates Calculate the percentage of water in sodium carbonate decahydrate, Na 2 CO 3 • 10 H 2 O. 1. Molar Mass of Na 2 CO 3 • 10 H 2 O Na = 2(22. 99) = 45. 98 C = 1(12. 01) = 12. 01 H = 20(1. 01) = 20. 2 O = 13(16. 00)= 208. 00 3. MM = 286. 2 2. Water 180. 2 g _______ x 100%= 62. 96 % 286. 2 g H = 20(1. 01) = 20. 2 O = 10(16. 00)= 160. 00 MM = 180. 2 or H = 2(1. 01) = 2. 02 O = 1(16. 00) = 16. 00 MM H 2 O = 18. 02 So… 10 H 2 O = 10(18. 02) = 180. 2

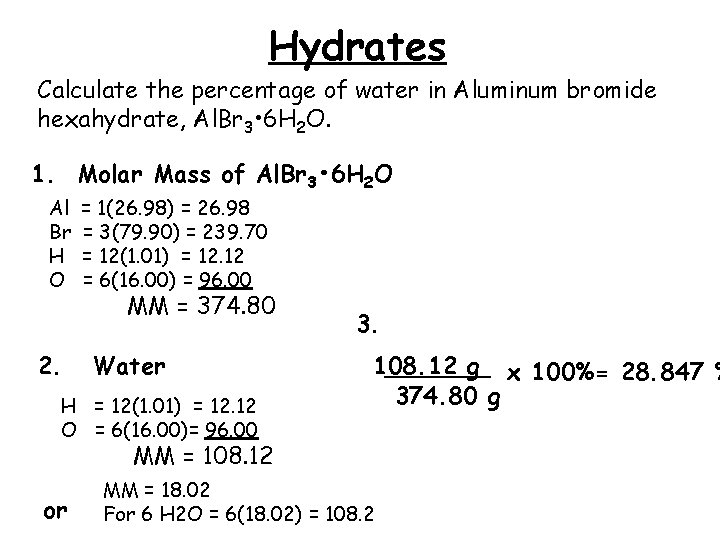
Hydrates Calculate the percentage of water in Aluminum bromide hexahydrate, Al. Br 3 • 6 H 2 O. 1. Molar Mass of Al. Br 3 • 6 H 2 O Al Br H O 2. = 1(26. 98) = 26. 98 = 3(79. 90) = 239. 70 = 12(1. 01) = 12. 12 = 6(16. 00) = 96. 00 MM = 374. 80 Water H = 12(1. 01) = 12. 12 O = 6(16. 00)= 96. 00 3. 108. 12 _______ g x 100%= 28. 847 % 374. 80 g MM = 108. 12 or MM = 18. 02 For 6 H 2 O = 6(18. 02) = 108. 2

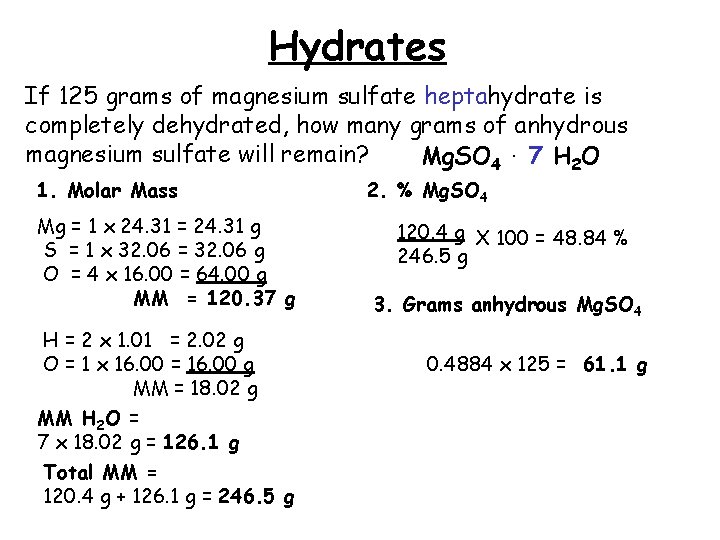
Hydrates If 125 grams of magnesium sulfate heptahydrate is completely dehydrated, how many grams of anhydrous magnesium sulfate will remain? Mg. SO 4. 7 H 2 O 1. Molar Mass Mg = 1 x 24. 31 = 24. 31 g S = 1 x 32. 06 = 32. 06 g O = 4 x 16. 00 = 64. 00 g MM = 120. 37 g H = 2 x 1. 01 = 2. 02 g O = 1 x 16. 00 = 16. 00 g MM = 18. 02 g MM H 2 O = 7 x 18. 02 g = 126. 1 g Total MM = 120. 4 g + 126. 1 g = 246. 5 g 2. % Mg. SO 4 120. 4 g X 100 = 48. 84 % 246. 5 g 3. Grams anhydrous Mg. SO 4 0. 4884 x 125 = 61. 1 g

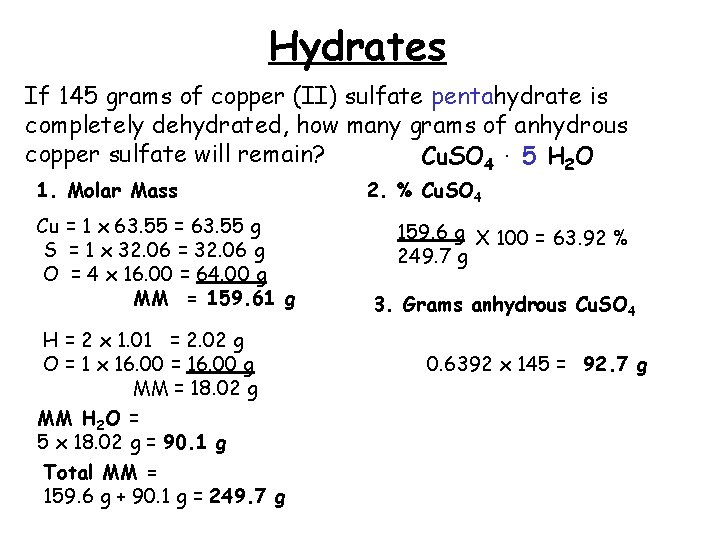
Hydrates If 145 grams of copper (II) sulfate pentahydrate is completely dehydrated, how many grams of anhydrous copper sulfate will remain? Cu. SO 4. 5 H 2 O 1. Molar Mass Cu = 1 x 63. 55 = 63. 55 g S = 1 x 32. 06 = 32. 06 g O = 4 x 16. 00 = 64. 00 g MM = 159. 61 g H = 2 x 1. 01 = 2. 02 g O = 1 x 16. 00 = 16. 00 g MM = 18. 02 g MM H 2 O = 5 x 18. 02 g = 90. 1 g Total MM = 159. 6 g + 90. 1 g = 249. 7 g 2. % Cu. SO 4 159. 6 g X 100 = 63. 92 % 249. 7 g 3. Grams anhydrous Cu. SO 4 0. 6392 x 145 = 92. 7 g

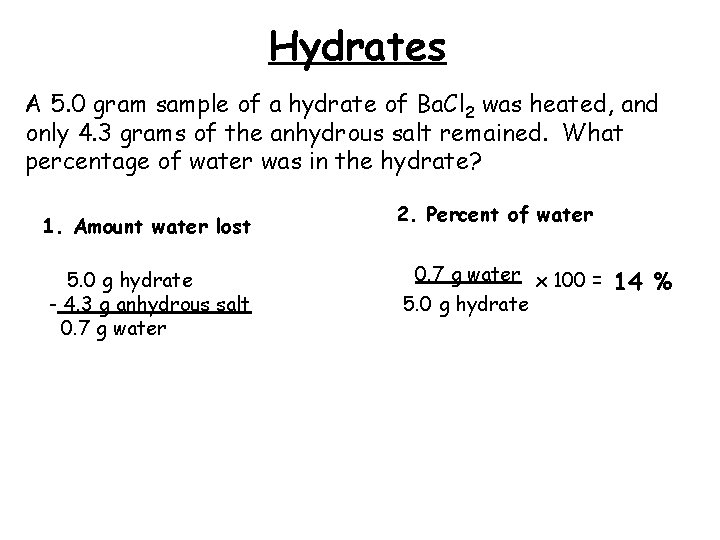
Hydrates A 5. 0 gram sample of a hydrate of Ba. Cl 2 was heated, and only 4. 3 grams of the anhydrous salt remained. What percentage of water was in the hydrate? 1. Amount water lost 5. 0 g hydrate - 4. 3 g anhydrous salt 0. 7 g water 2. Percent of water 0. 7 g water x 100 = 5. 0 g hydrate 14 %

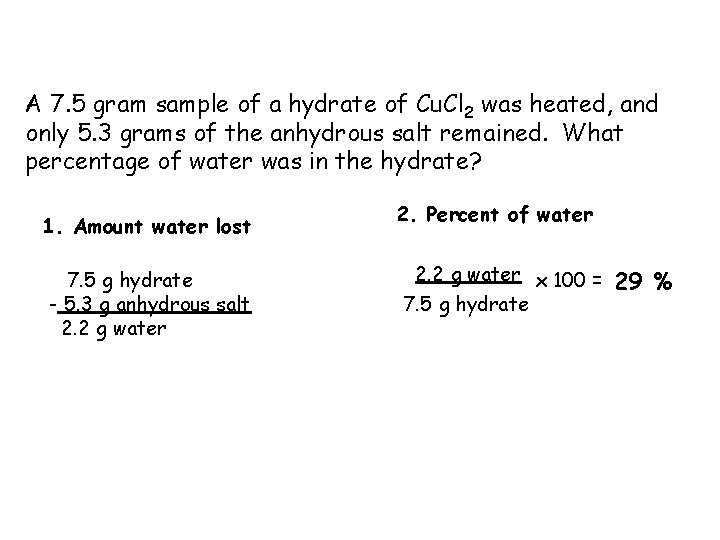
A 7. 5 gram sample of a hydrate of Cu. Cl 2 was heated, and only 5. 3 grams of the anhydrous salt remained. What percentage of water was in the hydrate? 1. Amount water lost 7. 5 g hydrate - 5. 3 g anhydrous salt 2. 2 g water 2. Percent of water 2. 2 g water x 100 = 7. 5 g hydrate 29 %

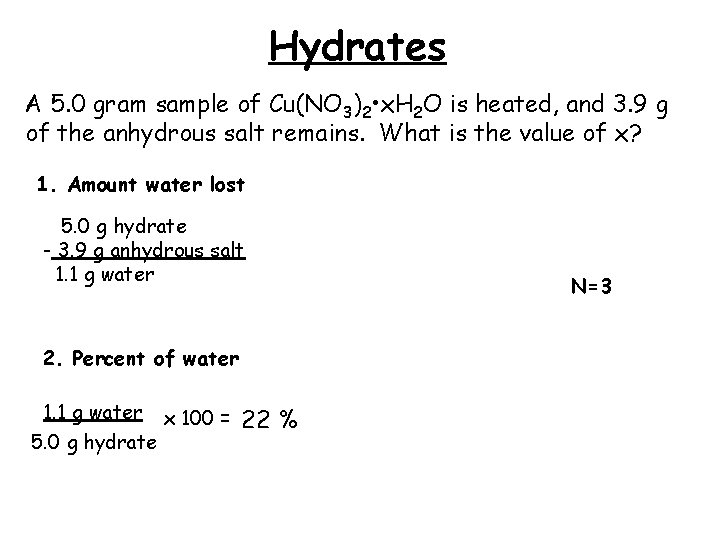
Hydrates A 5. 0 gram sample of Cu(NO 3)2 • x. H 2 O is heated, and 3. 9 g of the anhydrous salt remains. What is the value of x? 1. Amount water lost 5. 0 g hydrate - 3. 9 g anhydrous salt 1. 1 g water 2. Percent of water 1. 1 g water x 100 = 5. 0 g hydrate 22 % N=3

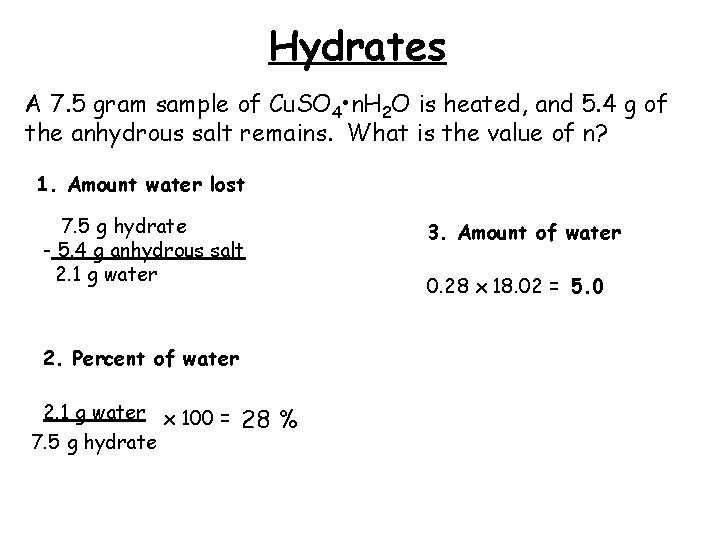
Hydrates A 7. 5 gram sample of Cu. SO 4 • n. H 2 O is heated, and 5. 4 g of the anhydrous salt remains. What is the value of n? 1. Amount water lost 7. 5 g hydrate - 5. 4 g anhydrous salt 2. 1 g water 2. Percent of water 2. 1 g water x 100 = 7. 5 g hydrate 28 % 3. Amount of water 0. 28 x 18. 02 = 5. 0

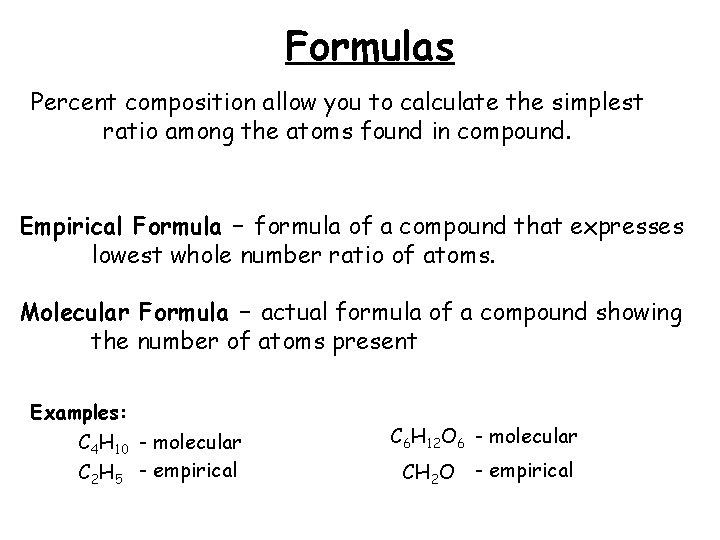
Formulas Percent composition allow you to calculate the simplest ratio among the atoms found in compound. Empirical Formula – formula of a compound that expresses lowest whole number ratio of atoms. Molecular Formula – actual formula of a compound showing the number of atoms present Examples: C 4 H 10 - molecular C 2 H 5 - empirical C 6 H 12 O 6 - molecular CH 2 O - empirical

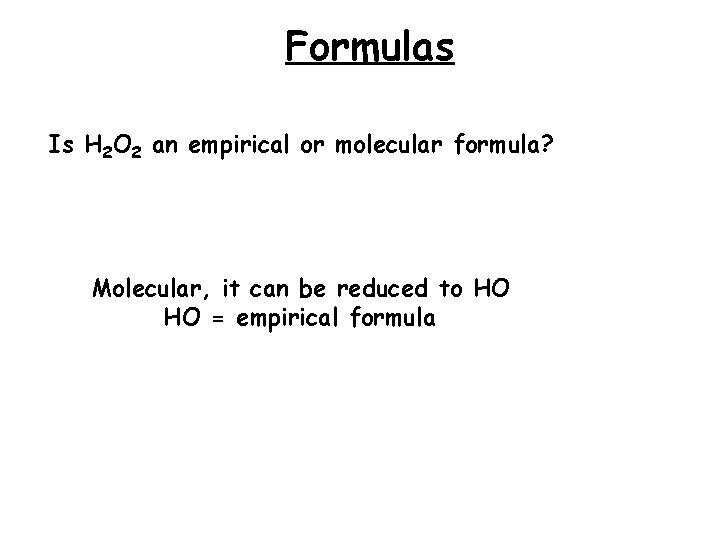
Formulas Is H 2 O 2 an empirical or molecular formula? Molecular, it can be reduced to HO HO = empirical formula

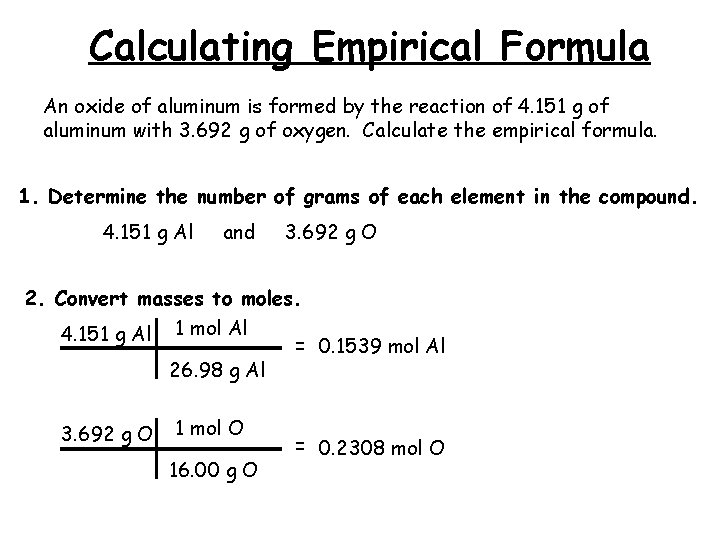
Calculating Empirical Formula An oxide of aluminum is formed by the reaction of 4. 151 g of aluminum with 3. 692 g of oxygen. Calculate the empirical formula. 1. Determine the number of grams of each element in the compound. 4. 151 g Al and 3. 692 g O 2. Convert masses to moles. 4. 151 g Al 1 mol Al = 0. 1539 mol Al 26. 98 g Al 3. 692 g O 1 mol O 16. 00 g O = 0. 2308 mol O

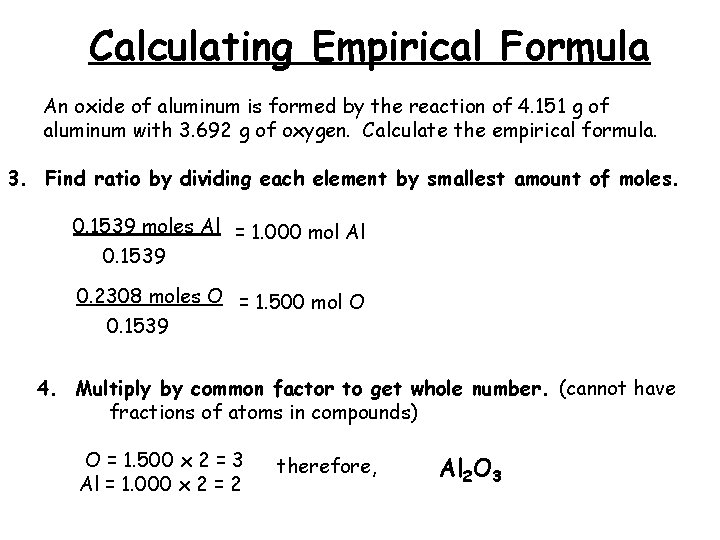
Calculating Empirical Formula An oxide of aluminum is formed by the reaction of 4. 151 g of aluminum with 3. 692 g of oxygen. Calculate the empirical formula. 3. Find ratio by dividing each element by smallest amount of moles. 0. 1539 moles Al = 1. 000 mol Al 0. 1539 0. 2308 moles O = 1. 500 mol O 0. 1539 4. Multiply by common factor to get whole number. (cannot have fractions of atoms in compounds) O = 1. 500 x 2 = 3 Al = 1. 000 x 2 = 2 therefore, Al 2 O 3

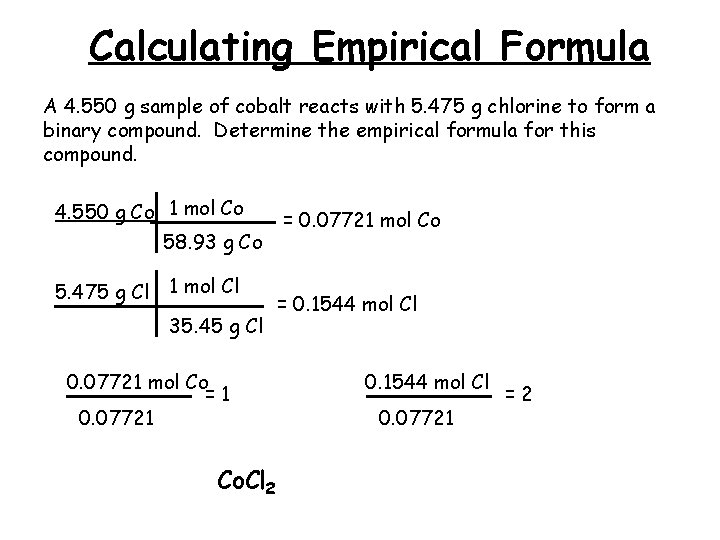
Calculating Empirical Formula A 4. 550 g sample of cobalt reacts with 5. 475 g chlorine to form a binary compound. Determine the empirical formula for this compound. 4. 550 g Co 1 mol Co 58. 93 g Co 5. 475 g Cl 1 mol Cl 35. 45 g Cl 0. 07721 mol Co =1 0. 07721 Co. Cl 2 = 0. 07721 mol Co = 0. 1544 mol Cl 0. 07721 =2

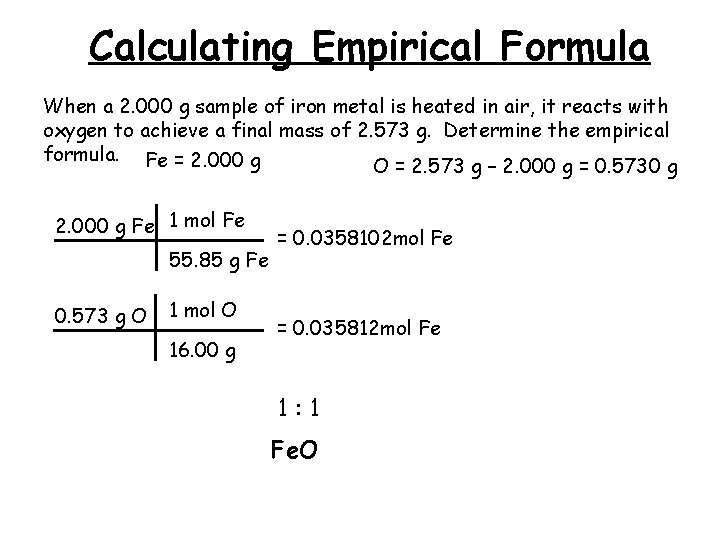
Calculating Empirical Formula When a 2. 000 g sample of iron metal is heated in air, it reacts with oxygen to achieve a final mass of 2. 573 g. Determine the empirical formula. Fe = 2. 000 g O = 2. 573 g – 2. 000 g = 0. 5730 g 2. 000 g Fe 1 mol Fe 55. 85 g Fe 0. 573 g O 1 mol O 16. 00 g = 0. 0358102 mol Fe = 0. 035812 mol Fe 1: 1 Fe. O

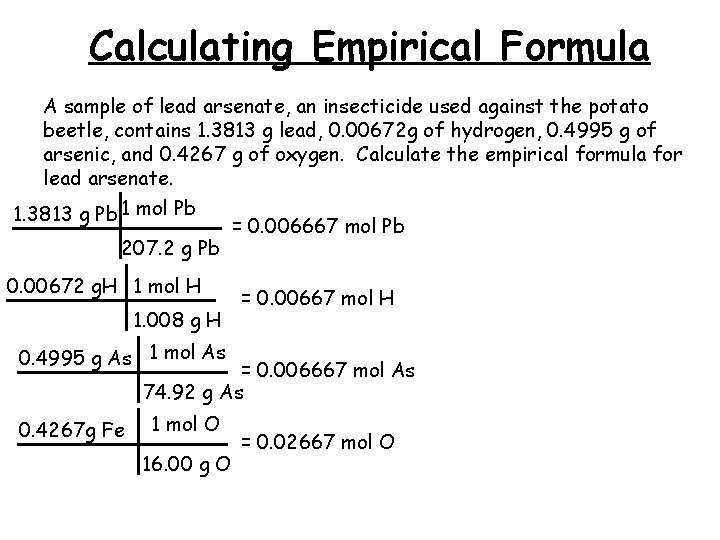
Calculating Empirical Formula A sample of lead arsenate, an insecticide used against the potato beetle, contains 1. 3813 g lead, 0. 00672 g of hydrogen, 0. 4995 g of arsenic, and 0. 4267 g of oxygen. Calculate the empirical formula for lead arsenate. 1. 3813 g Pb 1 mol Pb 207. 2 g Pb 0. 00672 g. H 1 mol H 1. 008 g H = 0. 006667 mol Pb = 0. 00667 mol H 0. 4995 g As 1 mol As = 0. 006667 mol As 74. 92 g As 0. 4267 g Fe 1 mol O 16. 00 g O = 0. 02667 mol O

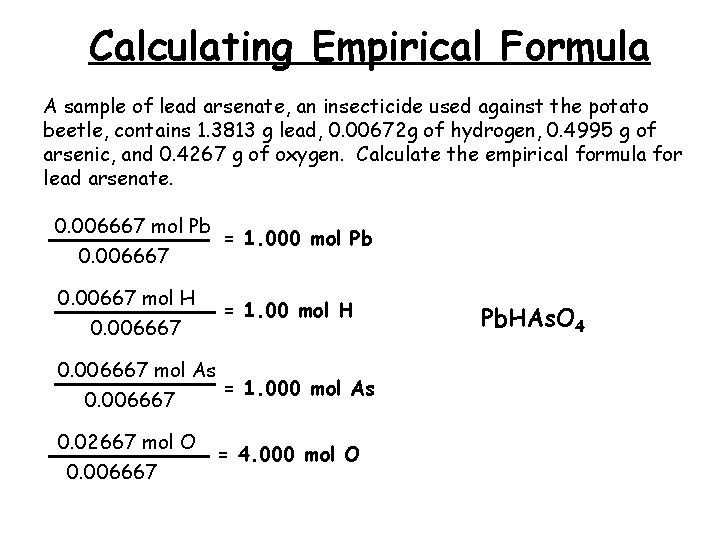
Calculating Empirical Formula A sample of lead arsenate, an insecticide used against the potato beetle, contains 1. 3813 g lead, 0. 00672 g of hydrogen, 0. 4995 g of arsenic, and 0. 4267 g of oxygen. Calculate the empirical formula for lead arsenate. 0. 006667 mol Pb = 1. 000 mol Pb 0. 006667 0. 00667 mol H 0. 006667 = 1. 00 mol H 0. 006667 mol As = 1. 000 mol As 0. 006667 0. 02667 mol O 0. 006667 = 4. 000 mol O Pb. HAs. O 4

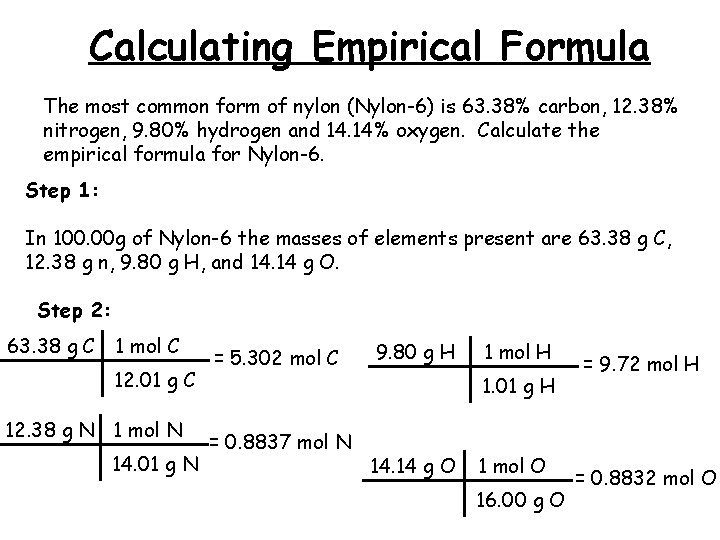
Calculating Empirical Formula The most common form of nylon (Nylon-6) is 63. 38% carbon, 12. 38% nitrogen, 9. 80% hydrogen and 14. 14% oxygen. Calculate the empirical formula for Nylon-6. Step 1: In 100. 00 g of Nylon-6 the masses of elements present are 63. 38 g C, 12. 38 g n, 9. 80 g H, and 14. 14 g O. Step 2: 63. 38 g C 1 mol C 12. 01 g C 12. 38 g N 1 mol N 14. 01 g N = 5. 302 mol C 9. 80 g H 1 mol H 1. 01 g H = 0. 8837 mol N 14. 14 g O 1 mol O 16. 00 g O = 9. 72 mol H = 0. 8832 mol O

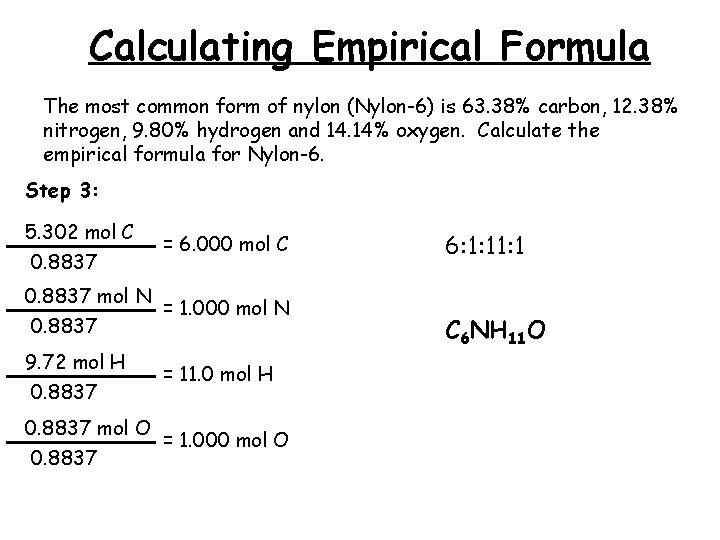
Calculating Empirical Formula The most common form of nylon (Nylon-6) is 63. 38% carbon, 12. 38% nitrogen, 9. 80% hydrogen and 14. 14% oxygen. Calculate the empirical formula for Nylon-6. Step 3: 5. 302 mol C 0. 8837 = 6. 000 mol C 0. 8837 mol N = 1. 000 mol N 0. 8837 9. 72 mol H 0. 8837 = 11. 0 mol H 0. 8837 mol O = 1. 000 mol O 0. 8837 6: 1: 1 C 6 NH 11 O

• If the molar mass is 339 g what is the molecular formula? • Factor =3 C 18 N 3 H 33 O 3

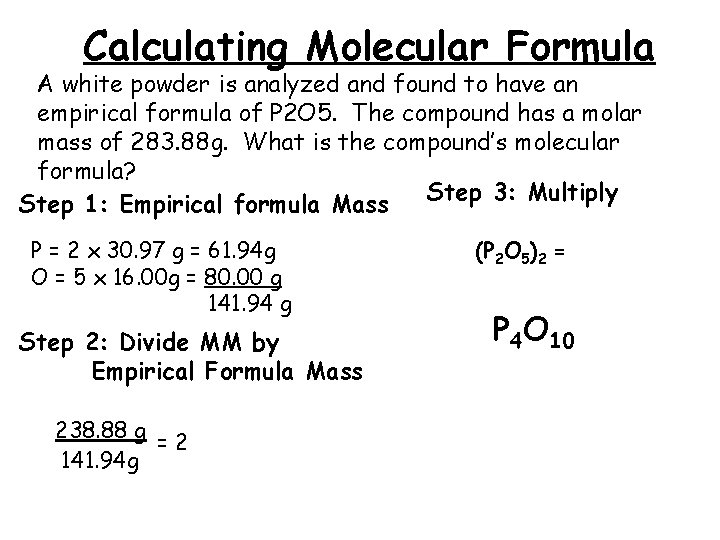
Calculating Molecular Formula A white powder is analyzed and found to have an empirical formula of P 2 O 5. The compound has a molar mass of 283. 88 g. What is the compound’s molecular formula? Step 3: Multiply Step 1: Empirical formula Mass P = 2 x 30. 97 g = 61. 94 g O = 5 x 16. 00 g = 80. 00 g 141. 94 g Step 2: Divide MM by Empirical Formula Mass 238. 88 g =2 141. 94 g (P 2 O 5)2 = P 4 O 10

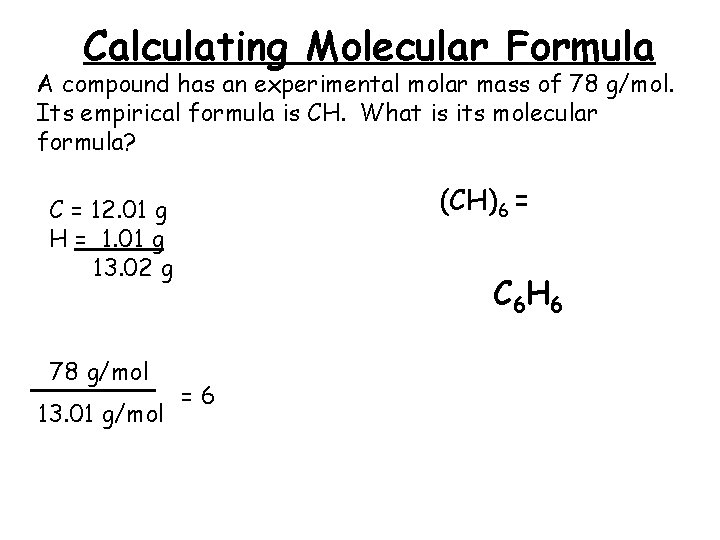
Calculating Molecular Formula A compound has an experimental molar mass of 78 g/mol. Its empirical formula is CH. What is its molecular formula? (CH)6 = C = 12. 01 g H = 1. 01 g 13. 02 g 78 g/mol 13. 01 g/mol C 6 H 6 =6