Percent Composition Empirical and Molecular Formulas Courtesy www

- Slides: 13

Percent Composition, Empirical and Molecular Formulas Courtesy www. lab-initio. com

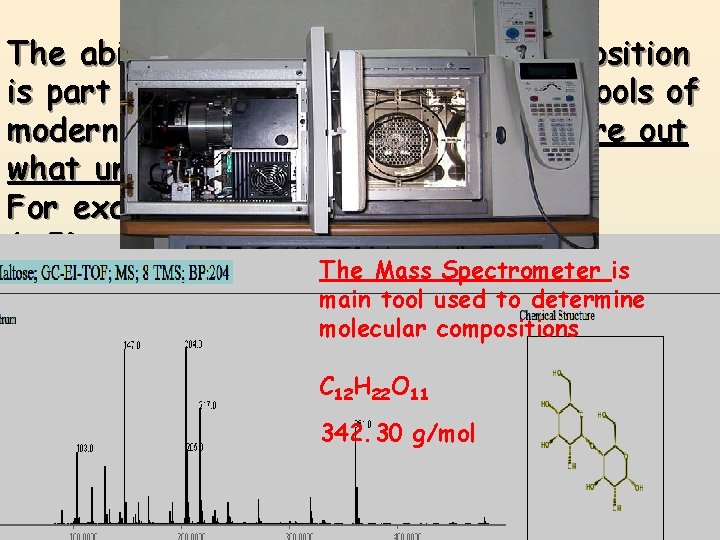
Percentage Composition The ability to calculate percent composition is part of one of the most powerful tools of modern science. Using it you can figure out what unknown substances are! For example: 1. ID poison or drugs someone has taken. The Mass Spectrometer is 2. Determine physical ailments, (liver, kidney main tool used to determine disease) based onmolecular the compounds compositionsin your blood or urine. C 12 H 22 O 11 3. ID chemical residue left at a crime scene 342. 30 g/mol or fire. 4. Determine valuable minerals in a rock

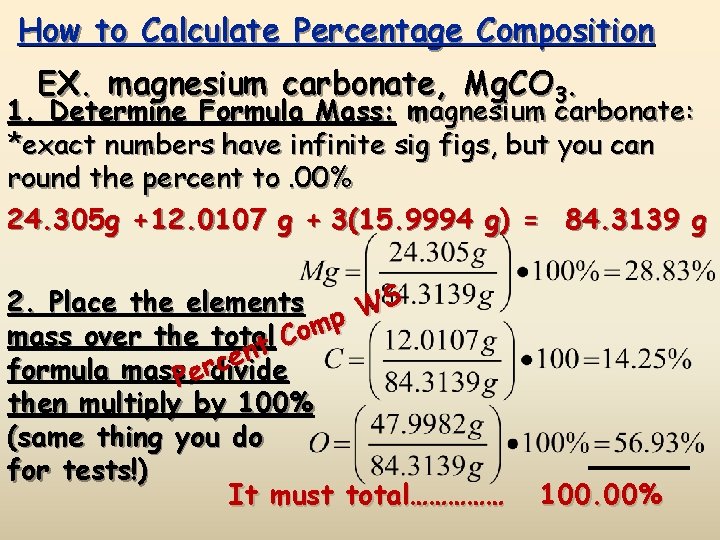
How to Calculate Percentage Composition EX. magnesium carbonate, Mg. CO 3. 1. Determine Formula Mass: magnesium carbonate: *exact numbers have infinite sig figs, but you can round the percent to. 00% 24. 305 g + 12. 0107 g + 3(15. 9994 g) = 84. 3139 g S 2. Place the elements W p m mass over the total Co t n e c formula mass, Perdivide then multiply by 100% (same thing you do for tests!) It must total…………… 100. 00%

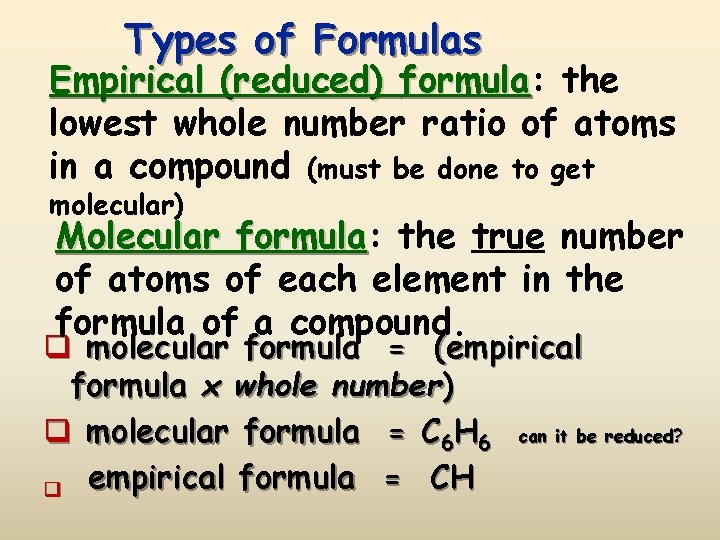
Types of Formulas Empirical (reduced) formula: formula the lowest whole number ratio of atoms in a compound (must be done to get molecular) Molecular formula: formula the true number of atoms of each element in the formula of a compound. q molecular formula = (empirical formula x whole number) q molecular formula = C 6 H 6 can it be reduced? q empirical formula = CH

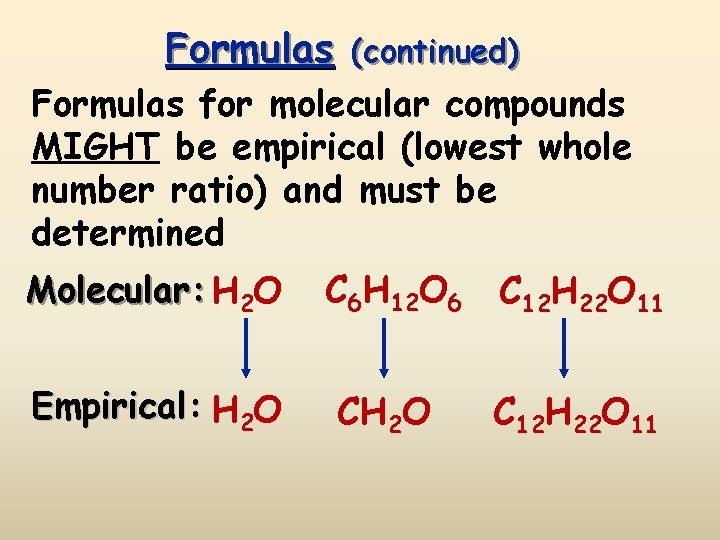
Formulas (continued) Formulas for molecular compounds MIGHT be empirical (lowest whole number ratio) and must be determined Molecular: H 2 O C 6 H 12 O 6 C 12 H 22 O 11 Empirical: H 2 O C 12 H 22 O 11

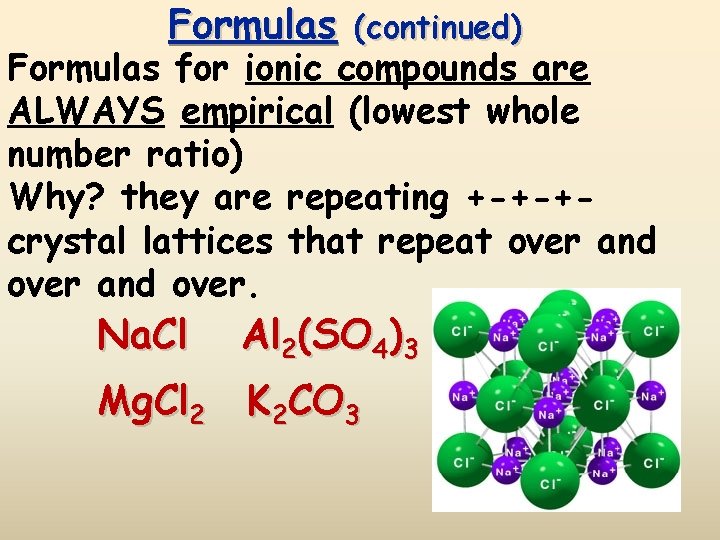
Formulas (continued) Formulas for ionic compounds are ALWAYS empirical (lowest whole number ratio) Why? they are repeating +-+-+crystal lattices that repeat over and over. Na. Cl Al 2(SO 4)3 Mg. Cl 2 K 2 CO 3

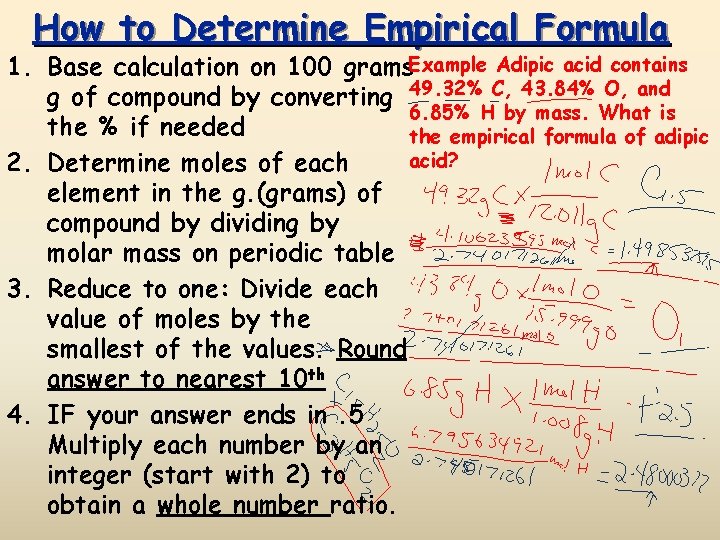
How to Determine Empirical Formula 1. Base calculation on 100 grams. Example Adipic acid contains g of compound by converting 49. 32% C, 43. 84% O, and 6. 85% H by mass. What is the % if needed the empirical formula of adipic acid? 2. Determine moles of each element in the g. (grams) of compound by dividing by molar mass on periodic table 3. Reduce to one: Divide each value of moles by the smallest of the values. Round answer to nearest 10 th 4. IF your answer ends in. 5 Multiply each number by an integer (start with 2) to obtain a whole number ratio.

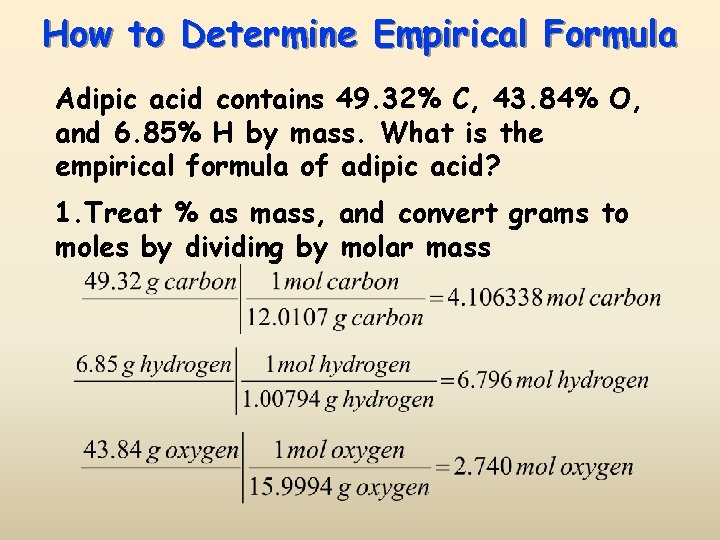
How to Determine Empirical Formula Adipic acid contains 49. 32% C, 43. 84% O, and 6. 85% H by mass. What is the empirical formula of adipic acid? 1. Treat % as mass, and convert grams to moles by dividing by molar mass

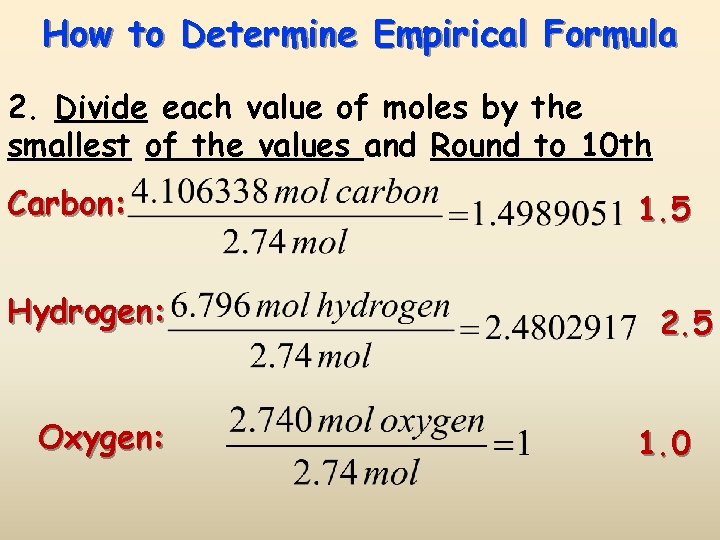
How to Determine Empirical Formula 2. Divide each value of moles by the smallest of the values and Round to 10 th Carbon: Hydrogen: Oxygen: 1. 5 2. 5 1. 0

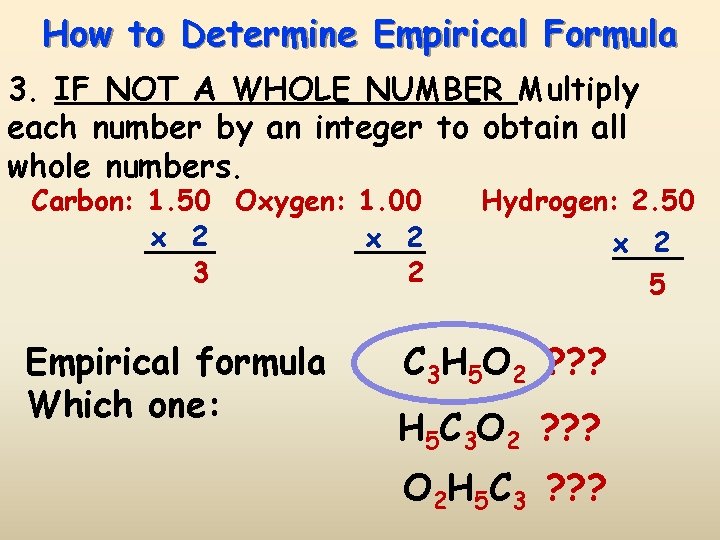
How to Determine Empirical Formula 3. IF NOT A WHOLE NUMBER Multiply each number by an integer to obtain all whole numbers. Carbon: 1. 50 Oxygen: 1. 00 x 2 3 2 Empirical formula Which one: Hydrogen: 2. 50 x 2 5 C 3 H 5 O 2 ? ? ? H 5 C 3 O 2 ? ? ? O 2 H 5 C 3 ? ? ?

Formula Order Rules: • No metals = Covalent: • 1. Carbon atoms first, • 2. Then hydrogen, • 3. Then other elements in alphabetical order BUT Oxygen is generally placed last. 4. If the formula contains NO carbon, then all of the elements are listed alphabetically. • When there is a metal/acid=Ionics: • Metallic positive ion first (usually least electronegative then non-metal elements or poly atomic ions. (you may need to ID Poly) • *lowest to highest electronegativity.

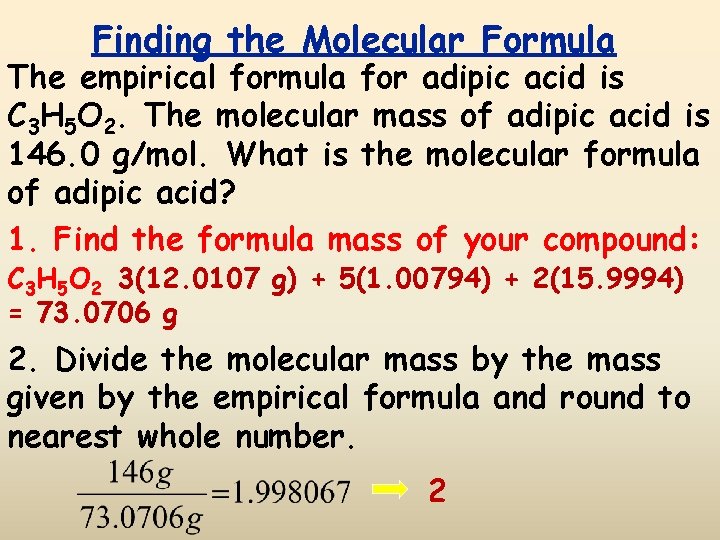
Finding the Molecular Formula The empirical formula for adipic acid is C 3 H 5 O 2. The molecular mass of adipic acid is 146. 0 g/mol. What is the molecular formula of adipic acid? 1. Find the formula mass of your compound: C 3 H 5 O 2 3(12. 0107 g) + 5(1. 00794) + 2(15. 9994) = 73. 0706 g 2. Divide the molecular mass by the mass given by the empirical formula and round to nearest whole number. 2

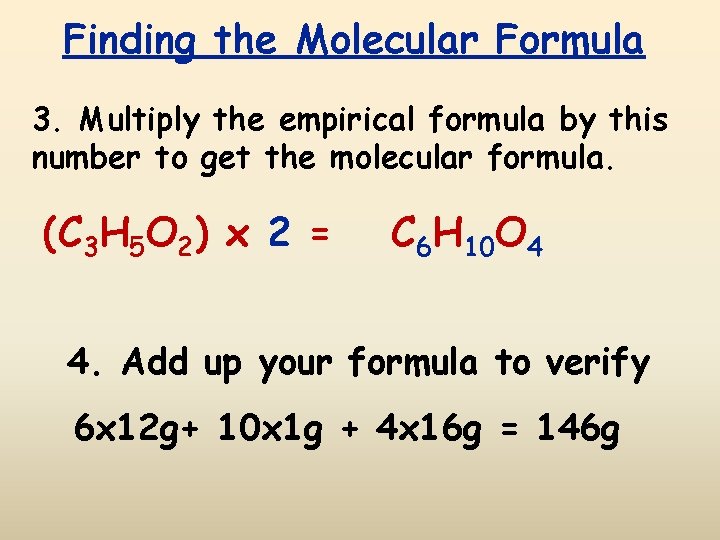
Finding the Molecular Formula 3. Multiply the empirical formula by this number to get the molecular formula. (C 3 H 5 O 2) x 2 = C 6 H 10 O 4 4. Add up your formula to verify 6 x 12 g+ 10 x 1 g + 4 x 16 g = 146 g