Percent Composition and chemical formulas Review and Essentials



- Slides: 10

Percent Composition and chemical formulas Review and Essentials

Percent Composition (percent by mass) is the relative amount of each element in a compound. • In order to calculate the percent composition of a known compound you need • Its molecular formula • Molar mass • The % mass is calculated for every element in the compound. If an element in the compound has a subscript that is counted in the mass of the element (ie. H 2 O, there are 2 hydrogen atoms, therefore the mass for hydrogen would be 2. 02 g)

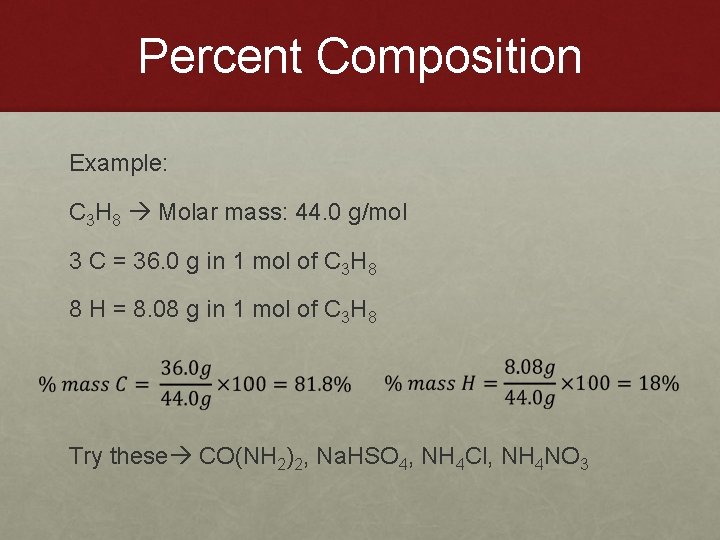
Percent Composition Example: C 3 H 8 Molar mass: 44. 0 g/mol 3 C = 36. 0 g in 1 mol of C 3 H 8 8 H = 8. 08 g in 1 mol of C 3 H 8 Try these CO(NH 2)2, Na. HSO 4, NH 4 Cl, NH 4 NO 3

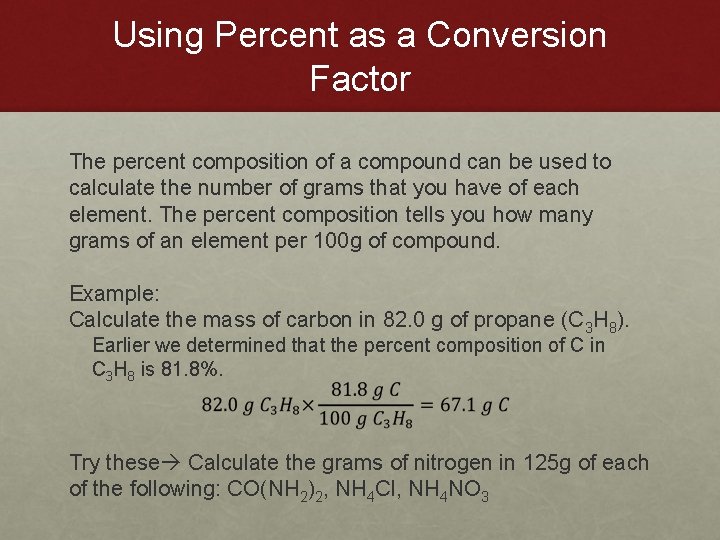
Using Percent as a Conversion Factor The percent composition of a compound can be used to calculate the number of grams that you have of each element. The percent composition tells you how many grams of an element per 100 g of compound. Example: Calculate the mass of carbon in 82. 0 g of propane (C 3 H 8). Earlier we determined that the percent composition of C in C 3 H 8 is 81. 8%. Try these Calculate the grams of nitrogen in 125 g of each of the following: CO(NH 2)2, NH 4 Cl, NH 4 NO 3

Calculating Empirical Formulas • With our newfound knowledge regarding percent composition we can calculate the empirical formula of a compound. • The empirical formula of a compound is the formula that gives us the lowest whole number ratio of moles of atoms that combine to form a compound (ie. The empirical formula for hydrogen peroxide is H 2 O 2 is HO). • The empirical formula can be the same as the molecular formula (ie. CO 2).

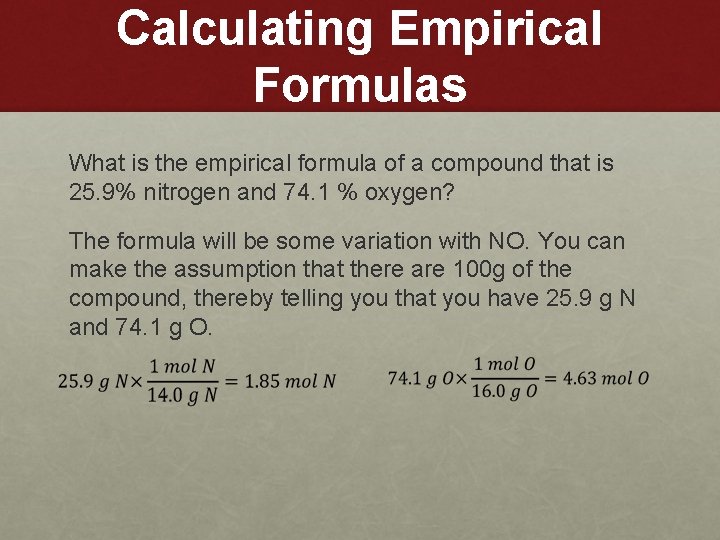
Calculating Empirical Formulas What is the empirical formula of a compound that is 25. 9% nitrogen and 74. 1 % oxygen? The formula will be some variation with NO. You can make the assumption that there are 100 g of the compound, thereby telling you that you have 25. 9 g N and 74. 1 g O.

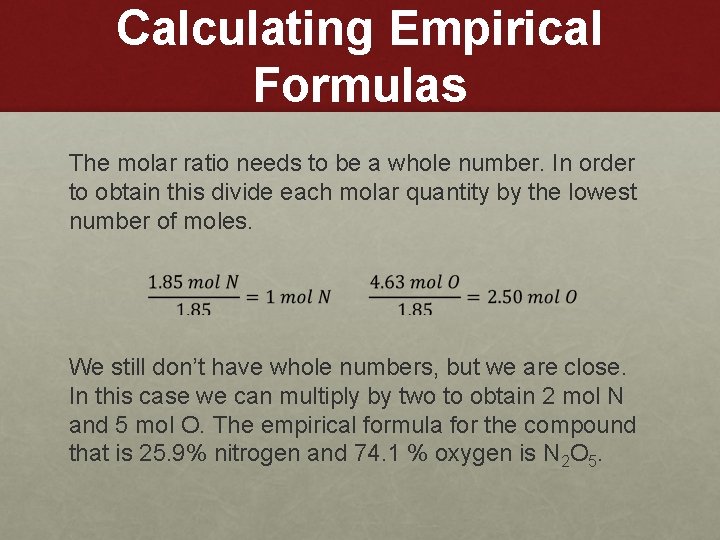
Calculating Empirical Formulas The molar ratio needs to be a whole number. In order to obtain this divide each molar quantity by the lowest number of moles. We still don’t have whole numbers, but we are close. In this case we can multiply by two to obtain 2 mol N and 5 mol O. The empirical formula for the compound that is 25. 9% nitrogen and 74. 1 % oxygen is N 2 O 5.

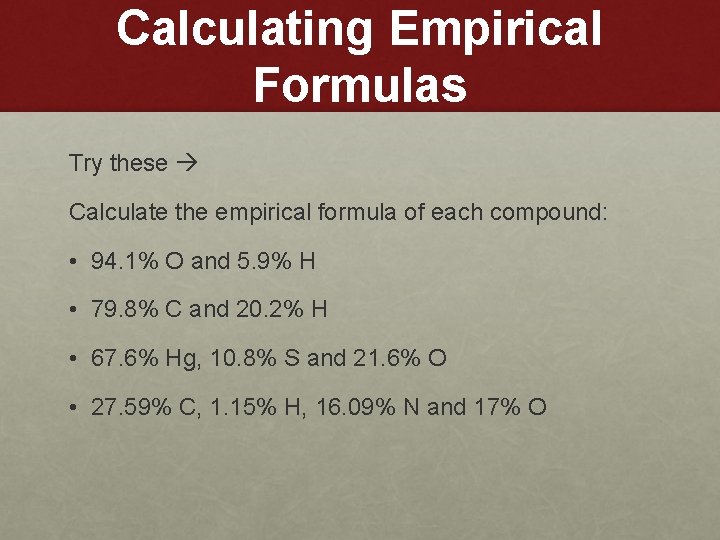
Calculating Empirical Formulas Try these Calculate the empirical formula of each compound: • 94. 1% O and 5. 9% H • 79. 8% C and 20. 2% H • 67. 6% Hg, 10. 8% S and 21. 6% O • 27. 59% C, 1. 15% H, 16. 09% N and 17% O

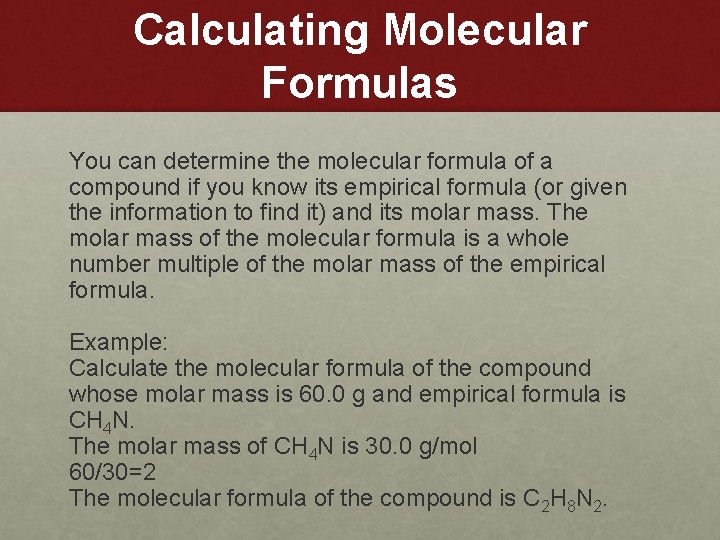
Calculating Molecular Formulas You can determine the molecular formula of a compound if you know its empirical formula (or given the information to find it) and its molar mass. The molar mass of the molecular formula is a whole number multiple of the molar mass of the empirical formula. Example: Calculate the molecular formula of the compound whose molar mass is 60. 0 g and empirical formula is CH 4 N. The molar mass of CH 4 N is 30. 0 g/mol 60/30=2 The molecular formula of the compound is C 2 H 8 N 2.

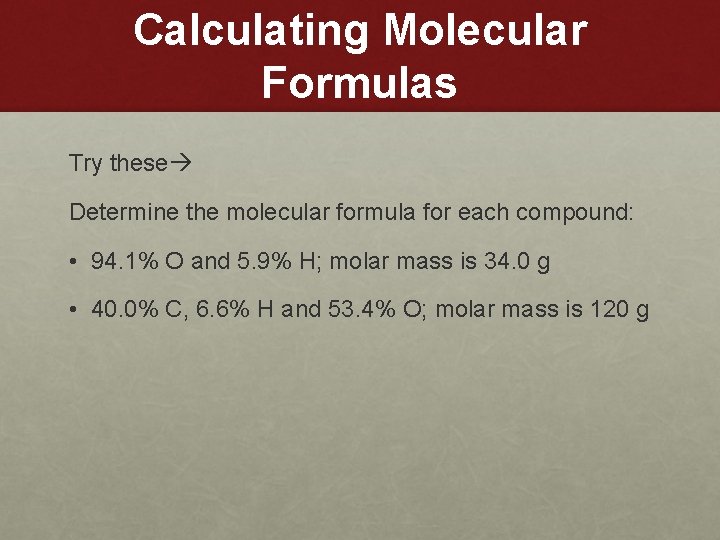
Calculating Molecular Formulas Try these Determine the molecular formula for each compound: • 94. 1% O and 5. 9% H; molar mass is 34. 0 g • 40. 0% C, 6. 6% H and 53. 4% O; molar mass is 120 g