Penny Density Lab Data Another lab that you

Penny Density Lab Data Another lab that you could have and would have done in class, but, all you really missed was measuring pennies on a scale, and then, putting these same pennies into water in a graduated cylinder to measure their volumes (to match with the masses) – so you could calculate the density of the 2 kinds of pennies (old ones vs. newer ones).

Why are we doing this lab? This is the first lab that has an “chemical point” to consider. It’s not just measuring, it’s measuring to discover something. If you don’t know why we are doing all of this measuring, then it’s a waste of time. This slide gives REASONS for you to do this work. From simple chemical principles (density, constants, and graphing, we can discover something you just don’t know yet. (how freaking cool is that!? ) So, old pennies are made of copper, which is an element. Details of copper are hiding in plain sight on Table S. The new pennies are only coated with copper on the outside, but a less expensive metal is inside, making up nearly 98% of the coin’s mass. The pennies are supposed to be exactly the same size (so they still fit into gumball machines). The first part is about seeing how close you can measure the old pennies to the actual density of copper, which is known. The second part is to determine the average density of the newer pennies, and then comparing this to the densities of other metals on Table S, to see if we can at least get close to the actual metal inside thin copper coating. Now, if you measure EXACTLY correct (mass and volume) you will get an average density of coins that are 2% copper and 98% something else. Getting your measure to be exactly the density of a metal on Table S means you mis-measured a bit. Getting close, and realizing that this process works, and will always work, that is the lesson. We’re hoping you get CLOSE to the metal inside the coins. It’s NOT silver, but is sure LOOKS like it. You could use an emery board or file to etch the edge of a new penny, and you will see a very different color metal appear almost immediately (the copper coating is really thin. Chemistry is so cool. You can do things already to help you discover unknowns, because elements have constant values that you can use and know about. Go…

Collecting the mass of the NEW PENNIES first. 9 = 20. 09 g 12 = 27. 50 g 15 = 35. 04 g 18 = 42. 60 g 21 = 50. 12 g

Collecting the mass of the NEW PENNIES first. 24 = 57. 71 g 27 = 65. 26 g 30 = 72. 73 g
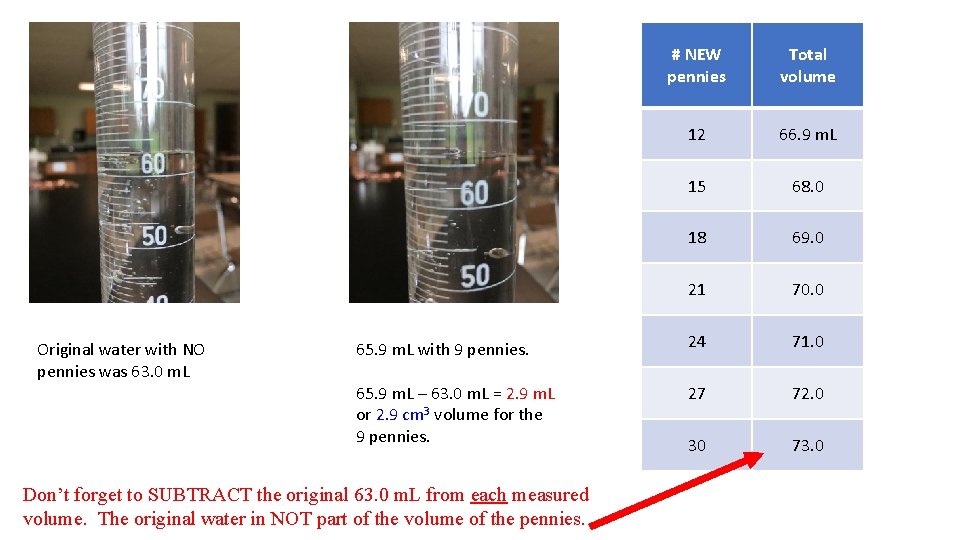
Original water with NO pennies was 63. 0 m. L # NEW pennies Total volume 12 66. 9 m. L 15 68. 0 18 69. 0 21 70. 0 65. 9 m. L with 9 pennies. 24 71. 0 65. 9 m. L – 63. 0 m. L = 2. 9 m. L or 2. 9 cm 3 volume for the 9 pennies. 27 72. 0 30 73. 0 Don’t forget to SUBTRACT the original 63. 0 m. L from each measured volume. The original water in NOT part of the volume of the pennies.

Collecting the mass of the OLD PENNIES second. 3 photos omitted data is good 9 = 27. 25 g 12 = 36. 50 g 15 = 45. 75 g 18 = 55. 08 g 21 = 64. 38 g

Collecting the mass of the OLD PENNIES second. 24 = 73. 68 g 27 = 82. 98 g 30 = 91. 71 g
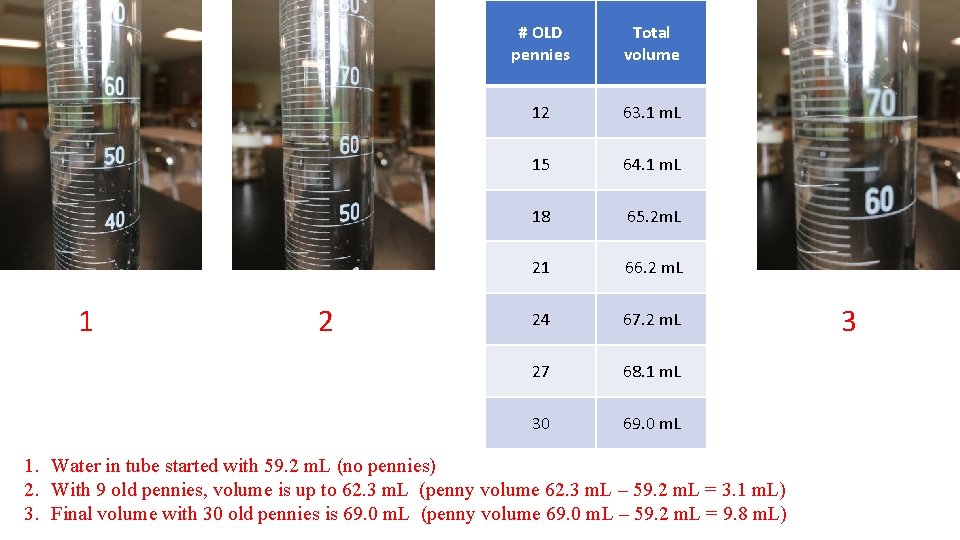
1 2 # OLD pennies Total volume 12 63. 1 m. L 15 64. 1 m. L 18 65. 2 m. L 21 66. 2 m. L 24 67. 2 m. L 27 68. 1 m. L 30 69. 0 m. L 1. Water in tube started with 59. 2 m. L (no pennies) 2. With 9 old pennies, volume is up to 62. 3 m. L (penny volume 62. 3 m. L – 59. 2 m. L = 3. 1 m. L) 3. Final volume with 30 old pennies is 69. 0 m. L (penny volume 69. 0 m. L – 59. 2 m. L = 9. 8 m. L) 3

We will not do the “average” the old fashioned way. Each data point (mass and volume) needs to be put into a graph with MASS as a FUNCTION of VOLUME. That means the graph has the MASS on the up and down Y axis The volumes go along the bottom, on the X axis. Do not mix this up or else you’re totally in the weeds. Your graph needs to be BIG, bigger graphs are better graphs. Mass in grams Your scales get NO BREAKS. Start at 0 grams and 0 cm 3. Make the scales consistent. Volume in cm 3 (same numerals as m. L)

Once you put ALL dots onto your two graphs, then we need to draw a best fit line, which MUST go through 0, 0. Your best fit line is your best attempt to average all of your points. The points let you draw the line, the line becomes your data. You may be making an error in the line placement, that is okay, as long as you understand that. Make sure your line is approximately through the center of your data points. Your line might not touch even one point. That’s okay too, the line is the “eyeball average” of your data points. It might hit one or more dots too, that is also okay. Mass in grams Volume in cm 3 (same numerals as m. L)

Once your best fit line is drawn, that line becomes your average measurement, representing ALL of your data. We need the slope of that line. The math is not hard, but your are likely less familiar with this than you think, we’ll talk about it in class (or read the lab handout). The slope of your line is the average of your measurements. Since slope is change of Y over change of X, and since Y is mass, and X is volume, the slope of this line will equal your average measured density of these pennies. Each graph has it’s own slope – which are not the same, the pennies are made of different metals (copper and then the unknown 98% metal). The slope of your old penny graph data should equal the density of copper from table S, since the old pennies are made up of 99. 9% copper. That makes sense. The other graph of new pennies has its own slope. That slope is the approximate density of the metal that is inside these new pennies. The slope (measured density) is not exactly the density of the new pennies, because they are made up of 2% copper and 98% of the unknown metal. But, your new penny graph slope should be CLOSE TO the densit of the metal inside the coins. Check.

READ THE RUBIC… This lab report needs a cover. The handout follows, then the TWO BIG GRAPHS, followed by the questions you still need to answer on white paper. The last page is the conclusion, which is worth 25% of the whole report, and is important. You will need to write and think, although thinking first is usually more better (haha). You will get through this, I promise. I know it’s hard too, but you’re big and I am a pretty good teacher, it will come together like Jello with fruit cocktail in it!
- Slides: 12