PEMFC Bipolar Plate Materials Process Hyeong Jin Jeon
PEMFC Bipolar Plate (Materials & Process) Hyeong Jin Jeon 2014. 05. 28. NEx. T MEMS Laboratory School of Mechanical Engineering Pusan National University
Contents 1. Introduction of PEMFC 2. Materials and Process of Bipolar Plate 3. Recent Trend of Bipolar Plate 2

1. Introduction of PEMFC
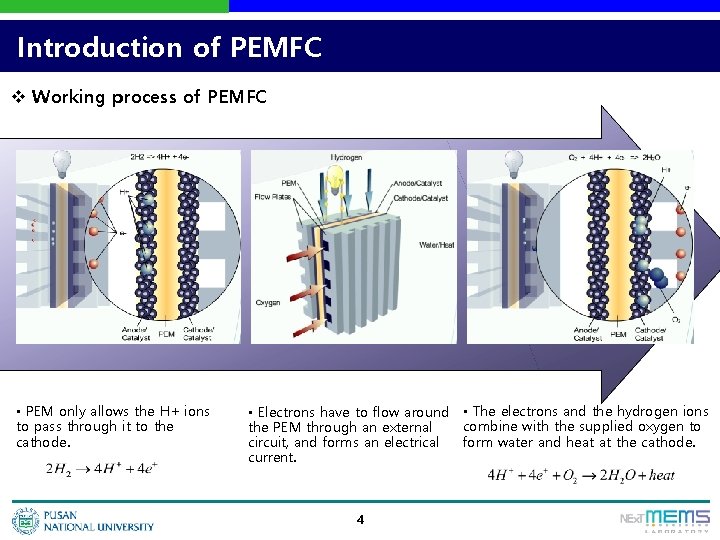
Introduction of PEMFC v Working process of PEMFC • PEM only allows the H+ ions to pass through it to the cathode. • Electrons have to flow around • The electrons and the hydrogen ions combine with the supplied oxygen to the PEM through an external form water and heat at the cathode. circuit, and forms an electrical current. 4
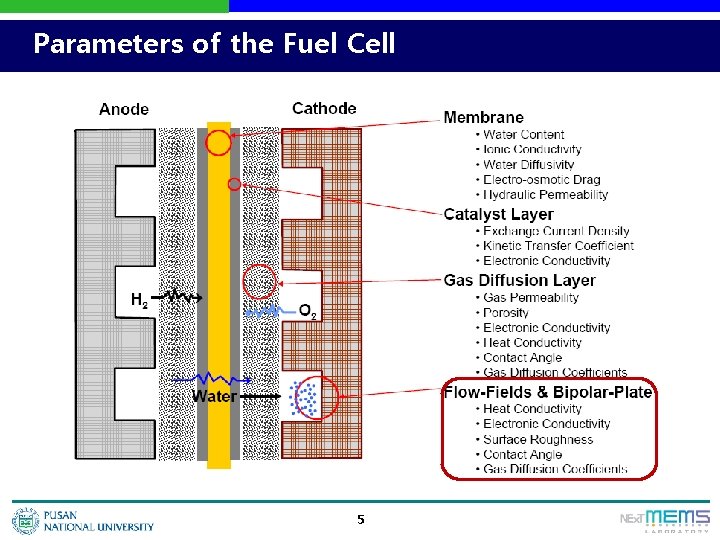
Parameters of the Fuel Cell 5

Stack Components of the Fuel Cell • Single cell Stack of cells (Bipolar plate needed) • PEMFC hardware consists of the Membrane Electrode Assembly(MEA), bipolar plate, seal and end plate. • Bipolar plate : One of the most costly and problematic of the fuel cell stack. • Suitable, low-cost bipolar plate materials becomes a key element of PEMFC stack development. Ref : V. Mehta, J. S. Cooper, J. Power Sources, 114, 32 (2003) 6

2. Materials and Process of Bipolar Plate
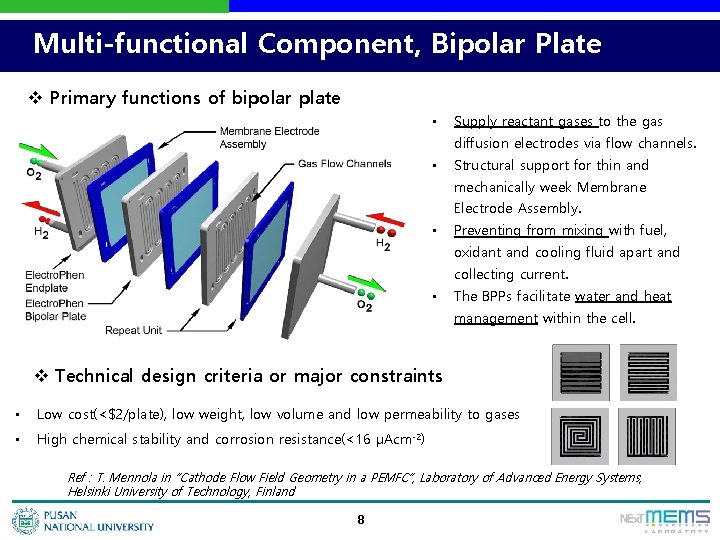
Multi-functional Component, Bipolar Plate v Primary functions of bipolar plate • Supply reactant gases to the gas diffusion electrodes via flow channels. • Structural support for thin and mechanically week Membrane Electrode Assembly. • Preventing from mixing with fuel, oxidant and cooling fluid apart and collecting current. • The BPPs facilitate water and heat management within the cell. v Technical design criteria or major constraints • Low cost(<$2/plate), low weight, low volume and low permeability to gases • High chemical stability and corrosion resistance(<16 μAcm-2) Ref : T. Mennola in “Cathode Flow Field Geometry in a PEMFC”, Laboratory of Advanced Energy Systems, Helsinki University of Technology, Finland 8
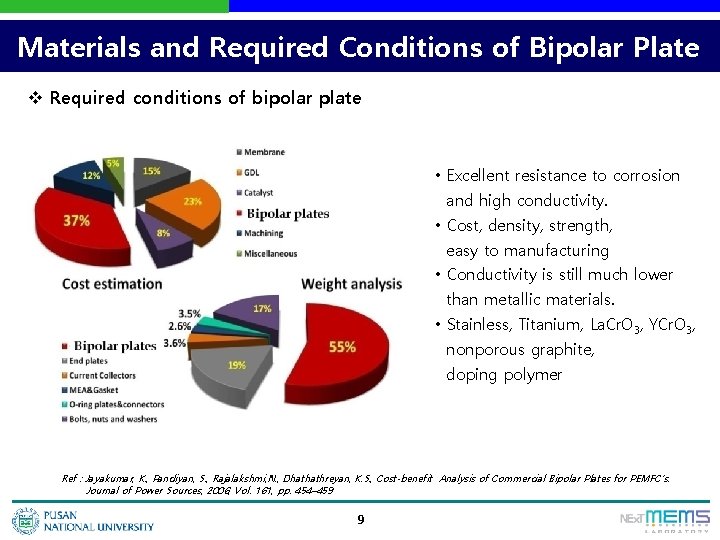
Materials and Required Conditions of Bipolar Plate v Required conditions of bipolar plate • Excellent resistance to corrosion and high conductivity. • Cost, density, strength, easy to manufacturing • Conductivity is still much lower than metallic materials. • Stainless, Titanium, La. Cr. O 3, YCr. O 3, nonporous graphite, doping polymer Ref : Jayakumar, K. , Pandiyan, S. , Rajalakshmi, N. , Dhathathreyan, K. S. , Cost-benefit Analysis of Commercial Bipolar Plates for PEMFC’s. Journal of Power Sources, 2006, Vol. 161, pp. 454– 459 9

Materials and Required Conditions of Bipolar Plate v Materials of bipolar plate • Stainless, Titanium, La. Cr. O 3, YCr. O 3, nonporous graphite, doping polymer • Graphite : High electrical and thermal conductivity, chemically inactivate, resistance to corrosion - Expensive to manufacturing - Mechanical machining, chemical etching : unsuitable for mass production • The greater part of PEMFC reduce size (3 mm) and weight Ref : Jayakumar, K. , Pandiyan, S. , Rajalakshmi, N. , Dhathathreyan, K. S. , Cost-benefit Analysis of Commercial Bipolar Plates for PEMFC’s. Journal of Power Sources, 2006, Vol. 161, pp. 454– 459 10

Bipolar Plate Materials and Names of Manufacturers Ref : “Bipolar plates for. PEMfuel cells: Areview”, Allen Hermann, International Journal of Hydrogen Energy 30, 2005, 1297 -1302 11
Metallic Bipolar Plate v Advantages of metallic bipolar plate • Resultant stack can be smaller and lighter than graphite bipolar plate. • Can be stamped to accommodate flow channels • Resultant plate can be varied thick, for example 100 ㎛. v Disadvantages of metallic bipolar plate • Susceptibility to corrosion and dissolution in the fuel cell operating environment of 80 ℃ and a p. H of 2 -3. • Harmful for cell performance. - Surface oxide creation significantly enlarges the contact resistance between the plate and GDL. - Corrosion process changes the morphology of the surface reducing the contact area. - Dissolved metal ions diffuse into the PEM membrane and become trapped. ü Non-coated metal alloys, precious non-coated metal ü Coated metals with a protective layer 12

Graphite Bipolar Plate v Advantages of graphite bipolar plate • Excellent chemical stability to survive the fuel cell environment. • Good resistance to corrosion. • Low bulk resistivity, low specific density and low electrical contact resistance with electrode backing materials. v Disadvantages of graphite bipolar plate • High costs. • Difficulty of machining it, its porosity. ü polymer/graphite composite • Low mechanical strength. Higher electrical and • Thick, high volume and weight. thermal conductivity. 13

Graphite and SS 316 Bipolar Plate v Comparison of the main properties between graphite and SS 316 • Performance comparison. • Coated SS 316 is a good replacement for graphite. • Cell performance of uncoated SS 316 is not satisfactory as graphite. • Conductivity, resistance of corrosion, heat expansion, micro crack, etc. Ref : X. Z. Yuan et al. / J. New Mat. Electrochem. Systems 8, 257 -267 (2005) 14

Polymer Composite Bipolar Plate v Advantages of polymer composite bipolar plate • Lightweight (metal-based / carbon-based) • Can be molded into any shape and size • Metal - based composite : Provides rigidity and chemical corrosion resistance. • Carbon - based composite : Using thermoplastic(polypropylene, polyethylene, poly(vinylidene fluoride) thermosetting resins (phenolics, epoxies and vinyl esters) v Research progress in graphite-polymer composites • Graphite – polymer composite : conductive composite material with excellent properties and a process for large scale production of bipolar plate. • Carbon composite : long term performances are comparable with graphite • Thermoplastic and carbon compound : injection molding of low-cost bipolar plates. • Vinyl ester – graphite composite : bulk-molding compound process Ref : “Bipolar plates for. PEMfuel cells: Areview”, Allen Hermann, International Journal of Hydrogen Energy 30, 2005, 1297 -1302 15

3. Recent Trend of Bipolar Plate - Sufficient corrosion resistance, Contact conductivity inexpensively

Conductive Polymer Composites v Development of conductive polymer composites(CPCs) • Polypropylene(PP) and poly(buthylene terephtalate)(PBT) composite • Crystalline natural graphite (10 -20 um flake-like particles) and carbon black (35 nm spherical particle) [Injection-molded] [Compression-molded] - Prevents electrons from flowing freely between the different layers of the sample Ref : Developing bipolar plates for fuel cels, Anett Kiraly and Ferenc Ronkay 17 • • CB (carbon black) G (graphite) PBT (poly(buthylene terephtalate) PP (Polypropylene)

Ti-Mo-N Film Coated on Stainless Steel v Mo content on microstructure • Stainless steel good thermal and electrical conductivity, mechanical properties • Cannot resist the corrosion of working condition • Ti-Mo-N film was fabricated on SS 316. • Enhanced corrosion resistance with different Mo contents. [Potentiodynamic polarization curves] Ref : Effects of Mo content on microstructure and corrosion resistance of arc ion plated Tie. Moe. N films on 316 L stainless steel as bipolar plates for polymer exchange membrane fuel cells, Min Zhang, Journal of Power Sources 253 (2014) 201 -204 18

Flexible Bipolar Plate v Special features & benefits • An unique feature of this bipolar plate is its flexibility • Due to high compression pressure, rigid bipolar plate imprints on the gas diffusion layer and damages the gas diffusion layer. • Plate with adequate flexibility reduces the deformation of gas diffusion layer. v Applications • Bipolar plate made with this technique covers wide range of size. • They can be used for fuel cell in various power range from 20 W to 5 k. W. • Applications include portable power sources, stationary power units, and distributed power generation. 19

Thank you for your attention!!
- Slides: 20