Pemeriksaan Terkini Hepatitis Virus Prof Dr Jusak Nugraha












- Slides: 53

Pemeriksaan Terkini Hepatitis Virus Prof. Dr. Jusak Nugraha, dr, MS, Sp. PK(K) Lab Patologi Klinik FK UNAIR

PENYAKIT HATI • Akut (kurang dari 6 bulan) • Kronik (lebih dari 6 bulan)

Virus penyebab Hepatitis • HAV : Hepatitis A virus • HBV : Hepatitis B virus • HCV : Hepatitis C virus • HDV : Hepatitis D virus • HEV : Hepatitis E virus ( penyebaran secara enterik di Asia dan epidemik ) • HGV : Hepatitis G virus • TTV : TT virus ( penularan melalui transfusi ) • SEN-V : non-ABCDE hepatitis ( penularan melalui transfusi ) 3

HEPATITISBINTHEWORLD • 2 billion people have been infected (1 out of 3 people). • 400 million people are chronically infected. • 10 -30 million will become infected each year. • An estimated 1 million people die each year from hepatitis B and its complications. • Approximately 2 people die each minute from hepatitis B. DR. T. V. RAO MD 4

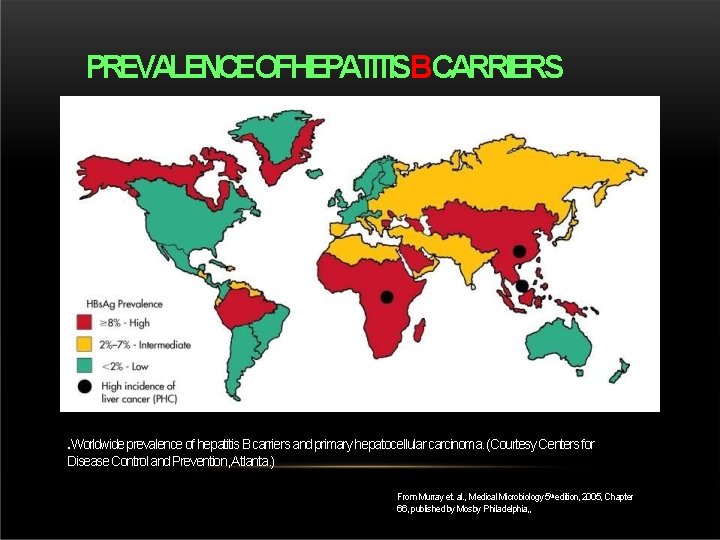
PREVALENCEOFHEPATITISBCARRIERS . Worldwide prevalence of hepatitis B carriers and primary hepatocellular carcinoma. (Courtesy Centers for Disease Control and Prevention, Atlanta. ) From Murray et. al. , Medical Microbiology 5 thedition, 2005, Chapter 66, published by Mosby Philadelphia, ,

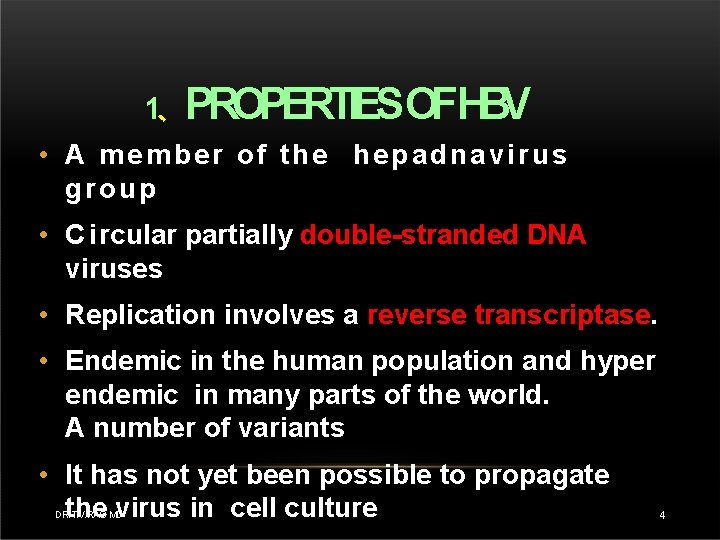
1、PROPERTIESOFHBV • A member of the hepadnavirus group • C i rcular partially double-stranded DNA viruses • Replication involves a reverse transcriptase. • Endemic in the human population and hyper endemic in many parts of the world. A number of variants • It has not yet been possible to propagate the virus in cell culture DR. T. V. RAO MD 4

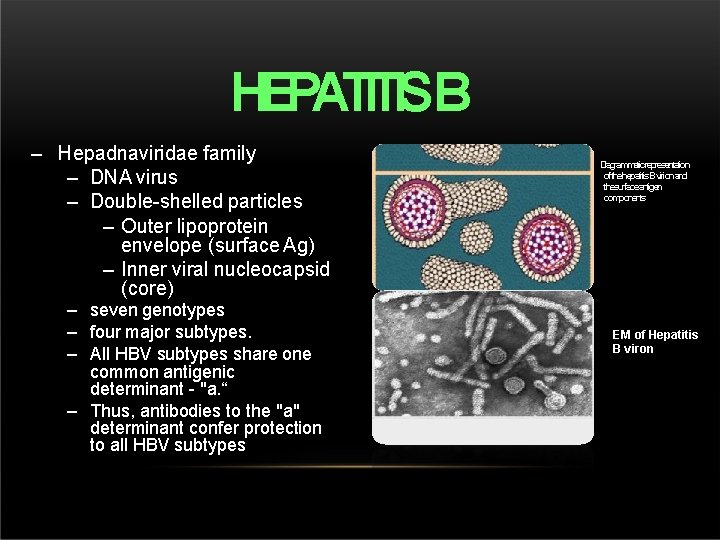
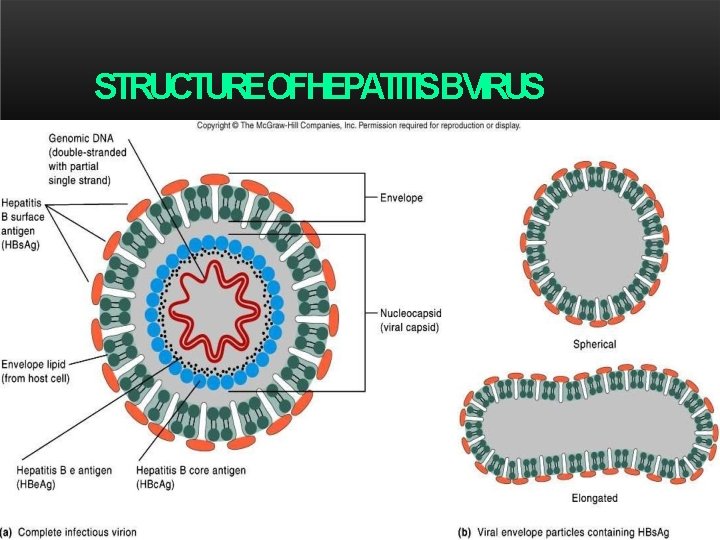
HEPATITISB – Hepadnaviridae family – DNA virus – Double-shelled particles – Outer lipoprotein envelope (surface Ag) – Inner viral nucleocapsid (core) – seven genotypes – four major subtypes. – All HBV subtypes share one common antigenic determinant - "a. “ – Thus, antibodies to the "a" determinant confer protection to all HBV subtypes Diagrammaticrepresentation ofthehepatitis. Bvirionand thesurfaceantigen components EM of Hepatitis B viron

HEPATITISBVIRUSAMAJORCAUSEOF HEPATITIS DR. T. V. RAO MD 8

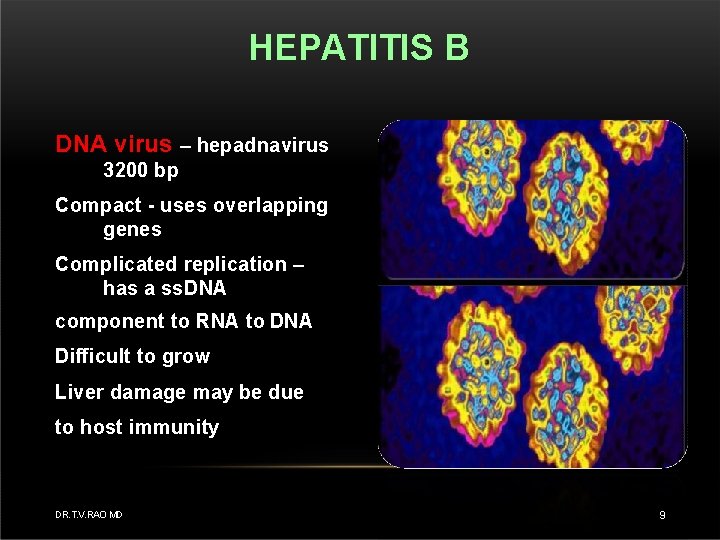
HEPATITIS B DNA virus – hepadnavirus 3200 bp Compact - uses overlapping genes Complicated replication – has a ss. DNA component to RNA to DNA Difficult to grow Liver damage may be due to host immunity DR. T. V. RAO MD 9

STRUCTUREOFHEPATITISBVIRUS

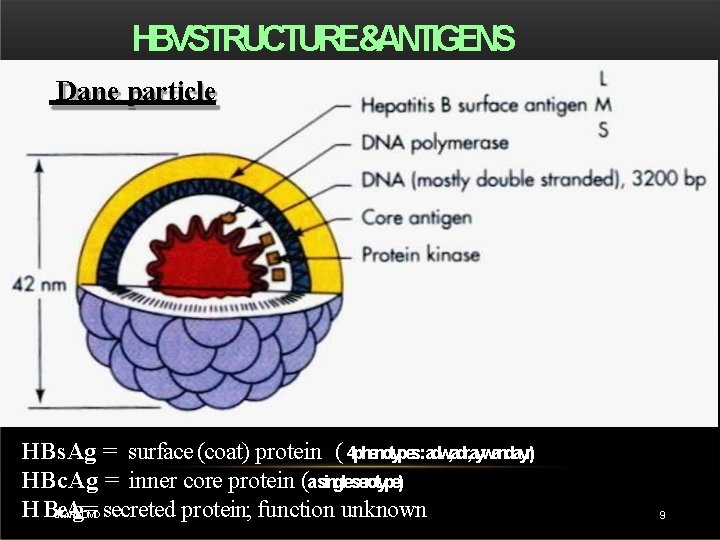
HBVSTRUCTURE&ANTIGENS Dane particle HBs. Ag = surface (coat) protein ( 4 phenotypes: adw, adr, aywandayr) HBc. Ag = inner core protein (asingleserotype) H BDRe. T. V. A g=MD secreted protein; function unknown. RAO 9

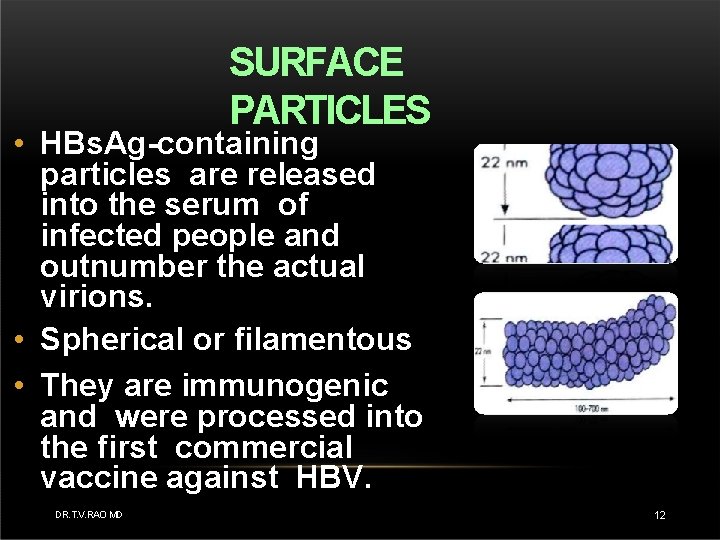
SURFACE PARTICLES • HBs. Ag-containing particles are released into the serum of infected people and outnumber the actual virions. • Spherical or filamentous • They are immunogenic and were processed into the first commercial vaccine against HBV. DR. T. V. RAO MD 12

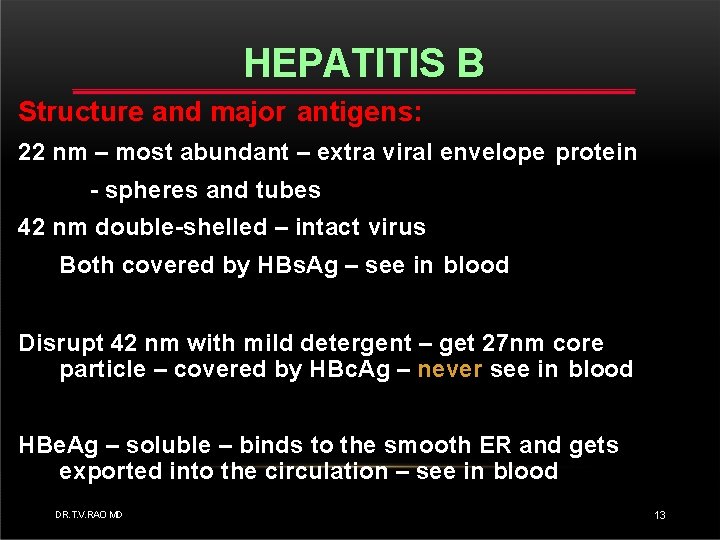
HEPATITIS B Structure and major antigens: 22 nm – most abundant – extra viral envelope protein - spheres and tubes 42 nm double-shelled – intact virus Both covered by HBs. Ag – see in blood Disrupt 42 nm with mild detergent – get 27 nm core particle – covered by HBc. Ag – never see in blood HBe. Ag – soluble – binds to the smooth ER and gets exported into the circulation – see in blood DR. T. V. RAO MD 13

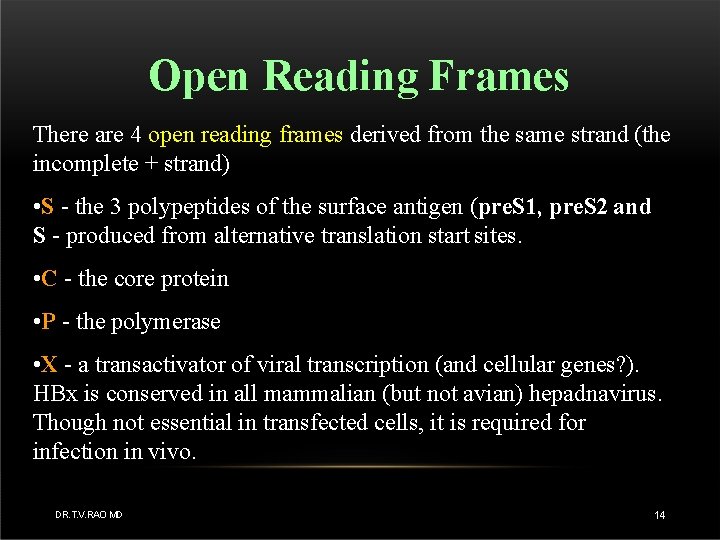
Open Reading Frames There are 4 open reading frames derived from the same strand (the incomplete + strand) • S - the 3 polypeptides of the surface antigen (pre. S 1, pre. S 2 and S - produced from alternative translation start sites. • C - the core protein • P - the polymerase • X - a transactivator of viral transcription (and cellular genes? ). HBx is conserved in all mammalian (but not avian) hepadnavirus. Though not essential in transfected cells, it is required for infection in vivo. DR. T. V. RAO MD 14

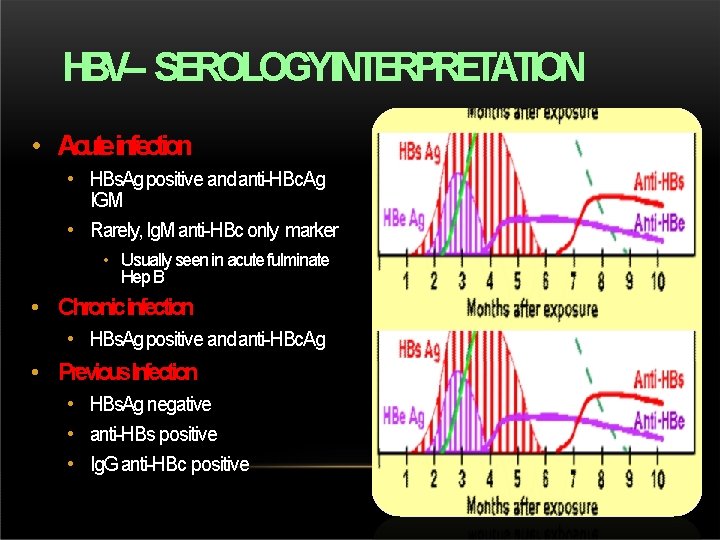
HBV– SEROLOGYINTERPRETATION • Acuteinfection • HBs. Ag positive and anti-HBc. Ag IGM • Rarely, Ig. M anti-HBc only marker • Usually seen in acute fulminate Hep B • Chronicinfection • HBs. Ag positive and anti-HBc. Ag • Previous. Infection • HBs. Ag negative • anti-HBs positive • Ig. G anti-HBc positive

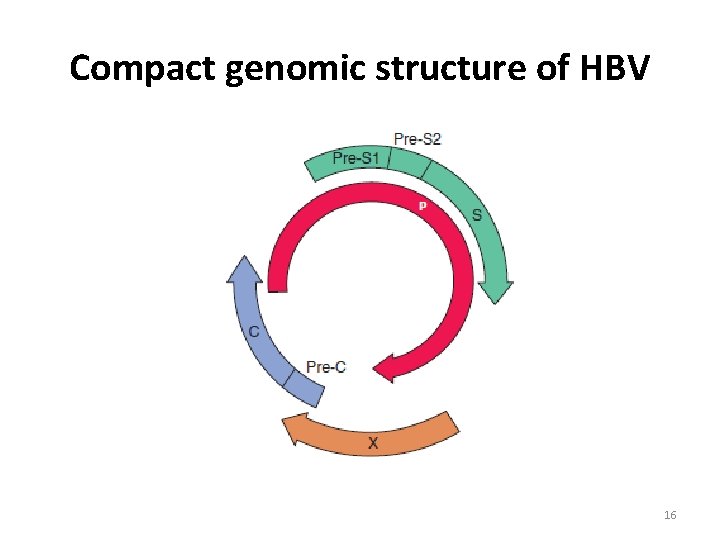
Compact genomic structure of HBV 16

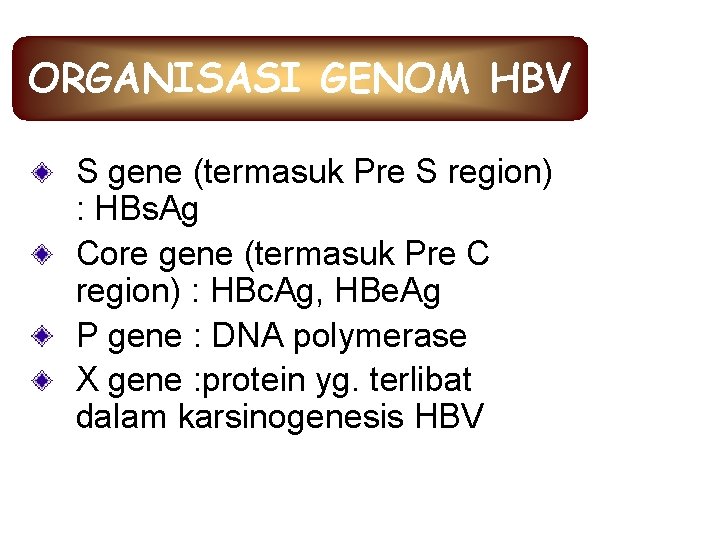
ORGANISASI GENOM HBV S gene (termasuk Pre S region) : HBs. Ag Core gene (termasuk Pre C region) : HBc. Ag, HBe. Ag P gene : DNA polymerase X gene : protein yg. terlibat dalam karsinogenesis HBV

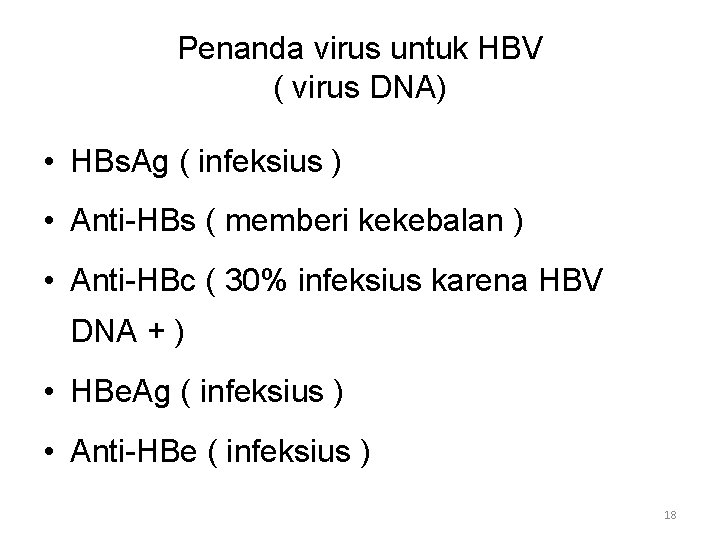
Penanda virus untuk HBV ( virus DNA) • HBs. Ag ( infeksius ) • Anti-HBs ( memberi kekebalan ) • Anti-HBc ( 30% infeksius karena HBV DNA + ) • HBe. Ag ( infeksius ) • Anti-HBe ( infeksius ) 18

Infeksi Hepatitis B Tes diagnostik laboratorium • Pemeriksaan serologi – Pemeriksaan antibodi sebagai respon imunologi tubuh yang terinfeksi virus : Anti HBc, Anti HBe, Anti HBs, Ig. M anti HBc, – Pemeriksaan antigen : HBs. Ag, HBe. Ag – Teknologi immunoassay: ELISA, EIA, ECLIA – Teknik Enzimchemiluminescence Immunoassay : teknologi immunoassay generasi terakhir (generasi 4) ; sensitivitas lebih baik dibandingkan generasi sebelumnya • Pemeriksaan muatan virus berbasis asam nukleat (HBV DNA) – Pemeriksaan muatan virus dengan metode PCR (Polymerase Chain Reaction) – Berbeda dengan immunoassay, memeriksa langsung kadar virus yang berhubungan dengan keaktifan virus berkembang dalam tubuh Hep. B_08

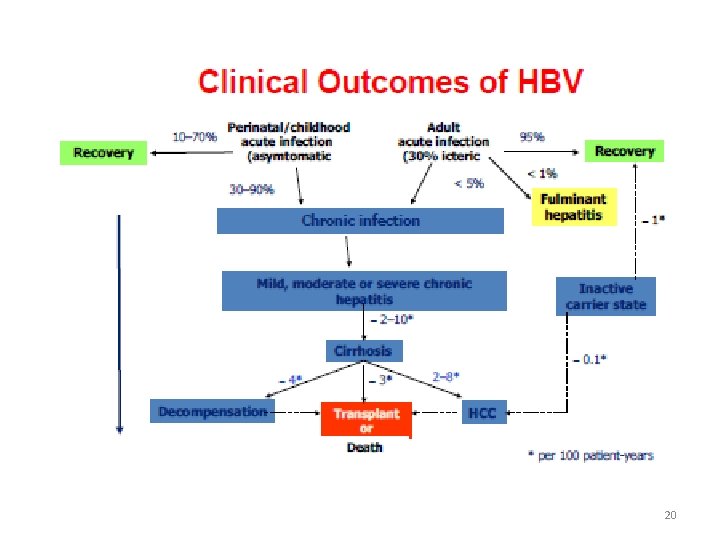
20

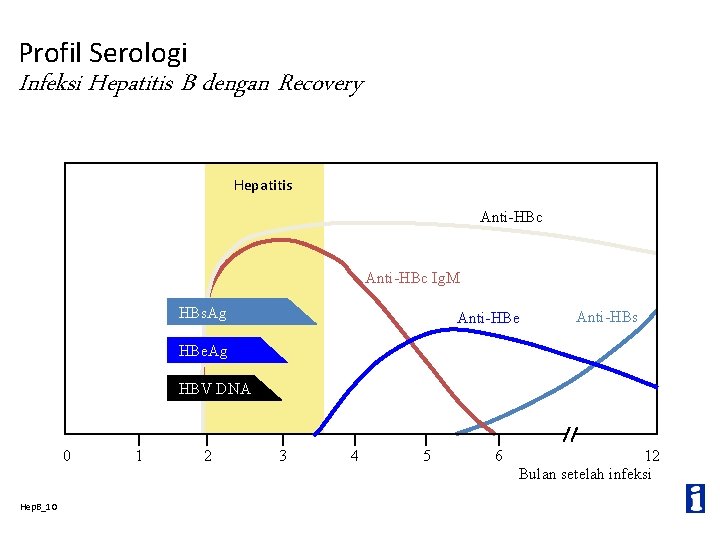
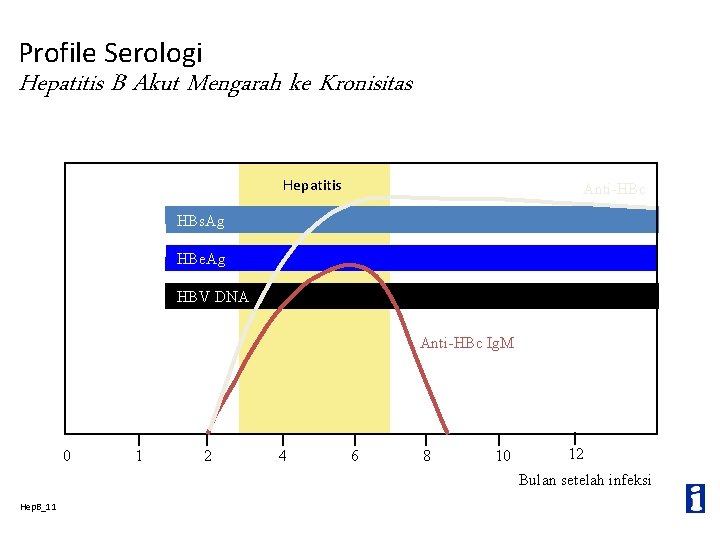
Profil Serologi Infeksi Hepatitis B dengan Recovery Hepatitis Anti-HBc Ig. M HBs. Ag Anti-HBe Anti-HBs HBe. Ag HBV DNA 0 Hep. B_10 1 2 3 4 5 6 12 Bulan setelah infeksi

Profile Serologi Hepatitis B Akut Mengarah ke Kronisitas Hepatitis Anti-HBc HBs. Ag HBe. Ag HBV DNA Anti-HBc Ig. M 0 1 2 4 6 8 10 12 Bulan setelah infeksi Hep. B_11

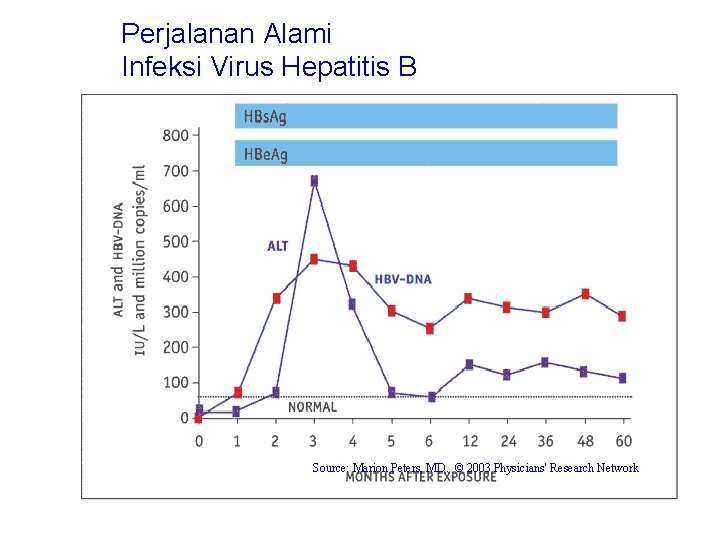
Perjalanan Alami Infeksi Virus Hepatitis B Source: Marion Peters, MD. © 2003 Physicians' Research Network

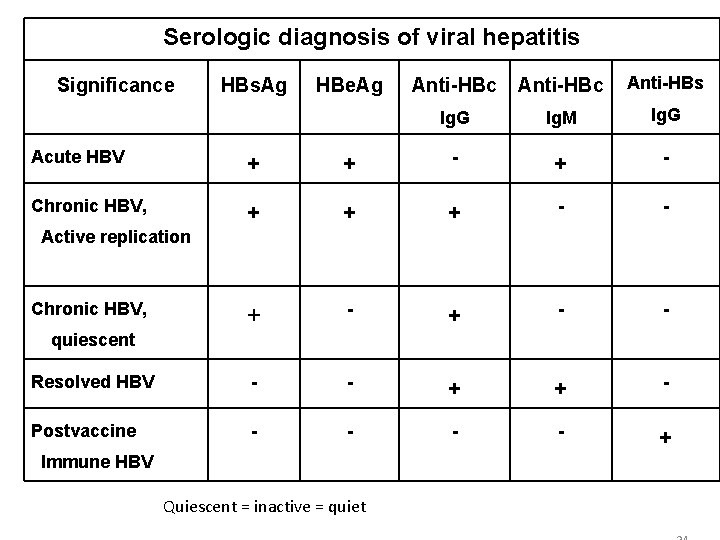
Serologic diagnosis of viral hepatitis Significance HBs. Ag HBe. Ag Anti-HBc Anti-HBs Ig. G Ig. M Ig. G Acute HBV + + - Chronic HBV, + + + - - Resolved HBV - - + + - Postvaccine - - + Active replication Chronic HBV, quiescent Immune HBV Quiescent = inactive = quiet

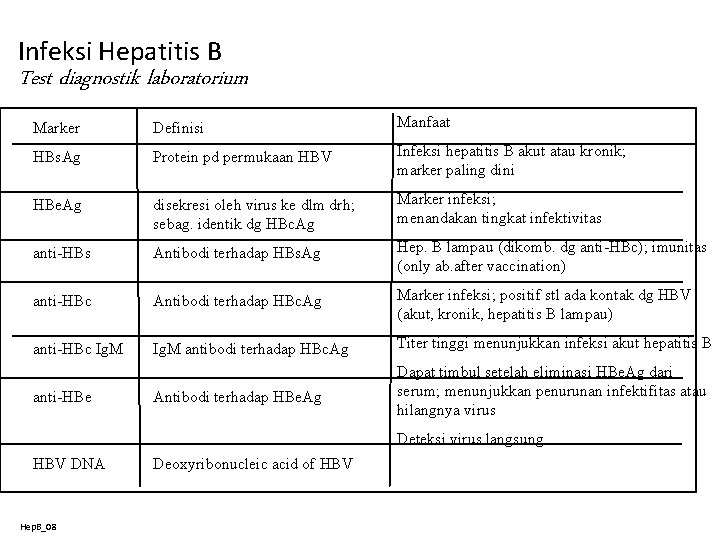
Infeksi Hepatitis B Test diagnostik laboratorium Marker Definisi Manfaat HBs. Ag Protein pd permukaan HBV Infeksi hepatitis B akut atau kronik; marker paling dini HBe. Ag disekresi oleh virus ke dlm drh; sebag. identik dg HBc. Ag Marker infeksi; menandakan tingkat infektivitas anti-HBs Antibodi terhadap HBs. Ag Hep. B lampau (dikomb. dg anti-HBc); imunitas (only ab. after vaccination) anti-HBc Antibodi terhadap HBc. Ag Marker infeksi; positif stl ada kontak dg HBV (akut, kronik, hepatitis B lampau) anti-HBc Ig. M antibodi terhadap HBc. Ag Titer tinggi menunjukkan infeksi akut hepatitis B Antibodi terhadap HBe. Ag Dapat timbul setelah eliminasi HBe. Ag dari serum; menunjukkan penurunan infektifitas atau hilangnya virus anti-HBe Deteksi virus langsung HBV DNA Hep. B_08 Deoxyribonucleic acid of HBV

• Pada kehamilan, transaminase serum dapat turun menjadi normal walau viremianya menetap, enzim tsb dapat meningkat lagi setelah kelahiran bayi. 26

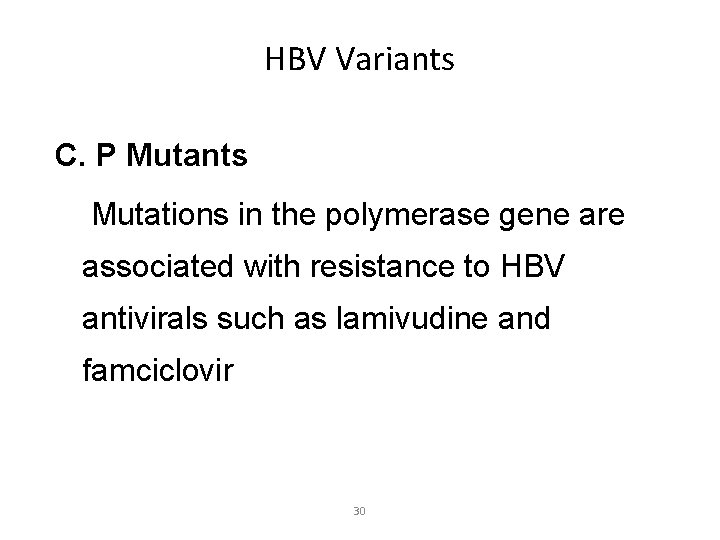
HBV Variants A. Precore mutants B. S Mutants C. P Mutants 27

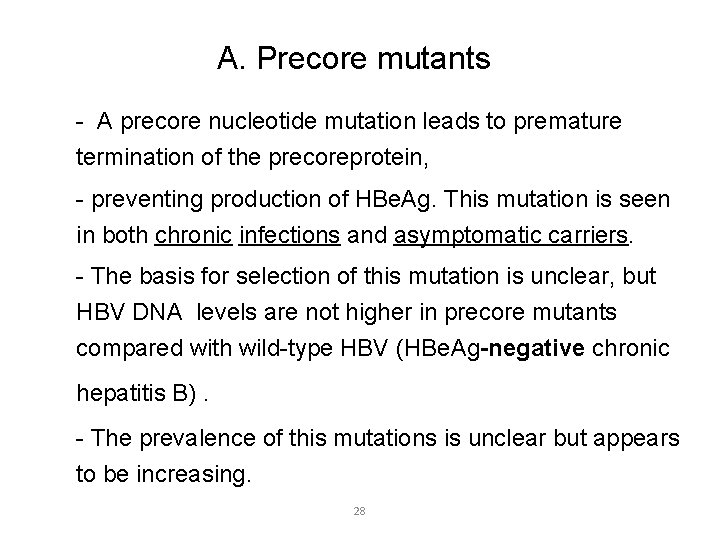
A. Precore mutants - A precore nucleotide mutation leads to premature termination of the precoreprotein, - preventing production of HBe. Ag. This mutation is seen in both chronic infections and asymptomatic carriers. - The basis for selection of this mutation is unclear, but HBV DNA levels are not higher in precore mutants compared with wild-type HBV (HBe. Ag-negative chronic hepatitis B). - The prevalence of this mutations is unclear but appears to be increasing. 28

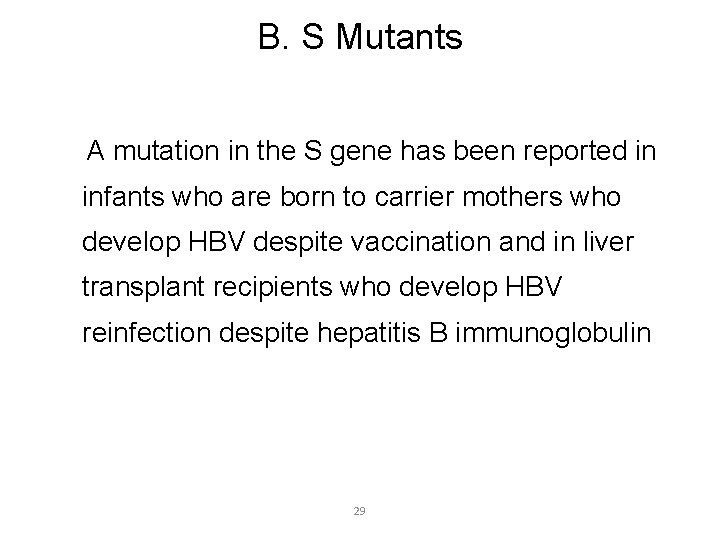
B. S Mutants A mutation in the S gene has been reported in infants who are born to carrier mothers who develop HBV despite vaccination and in liver transplant recipients who develop HBV reinfection despite hepatitis B immunoglobulin 29

HBV Variants C. P Mutants Mutations in the polymerase gene are associated with resistance to HBV antivirals such as lamivudine and famciclovir 30

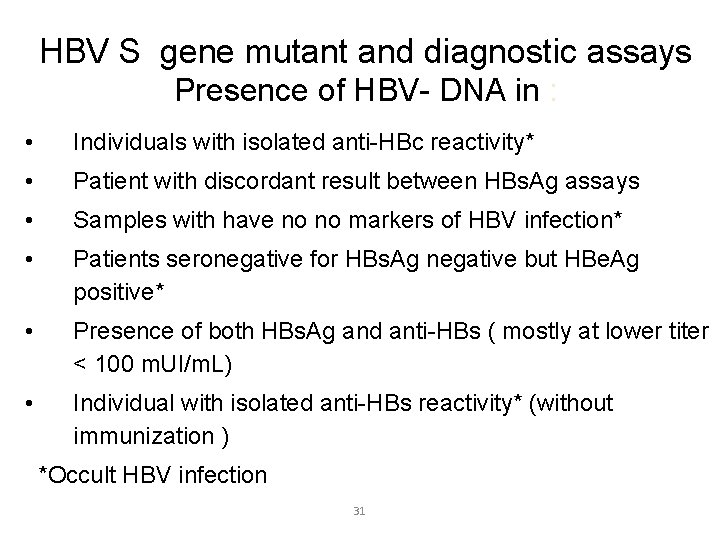
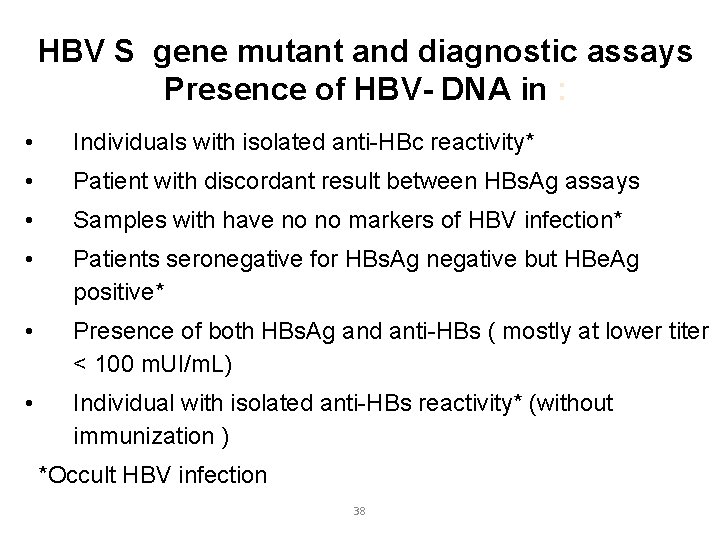
HBV S gene mutant and diagnostic assays Presence of HBV- DNA in : • Individuals with isolated anti-HBc reactivity* • Patient with discordant result between HBs. Ag assays • Samples with have no no markers of HBV infection* • Patients seronegative for HBs. Ag negative but HBe. Ag positive* • Presence of both HBs. Ag and anti-HBs ( mostly at lower titer < 100 m. UI/m. L) • Individual with isolated anti-HBs reactivity* (without immunization ) *Occult HBV infection 31

Chronic Hepatitis B Infection by a pre-core mutant of HBV or spontaneous mutation of the pre-core mutant or core promoter region of the HBV genome during the course of chronic hepatitis caused by wild-type HBV ( HBe. Ag negative chronic hepatitis B) may result in particularly severe chronic hepatitis with rapid progression to cirrhosis ( 8 -10%/year ) particularly + additional mutations in the core gene of HBV 32

Chronic Hepatitis B (2) The risk of cirrhosis and HCC correlates with the baseline serum HBV DNA level in Px with either HBe. Ag-positive or HBe. Ag-negative 33

Hepatitis B HBV DNA ( Roche, quantitative ) Reference : ND < 300 copies/ m. L Method : PCR, Hybridisation Clinical Usage : For detection and quantitative measurement of hepatitis B viral DNA in human serum. Assay able to detect low grade viremia, down to 300 copies/m. L but the upper detection limit is only at 2 x 10 5 copies/m. L 34

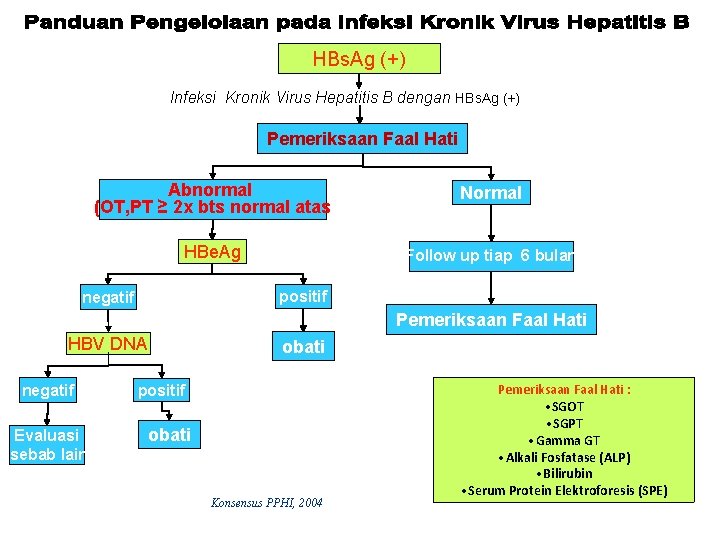
HBs. Ag (+) Infeksi Kronik Virus Hepatitis B dengan HBs. Ag (+) Pemeriksaan Faal Hati Abnormal (OT, PT ≥ 2 x bts normal atas Normal HBe. Ag Follow up tiap 6 bulan positif negatif Pemeriksaan Faal Hati HBV DNA negatif Evaluasi sebab lain obati positif obati Konsensus PPHI, 2004 Pemeriksaan Faal Hati : • SGOT • SGPT • Gamma GT • Alkali Fosfatase (ALP) • Bilirubin • Serum Protein Elektroforesis (SPE)

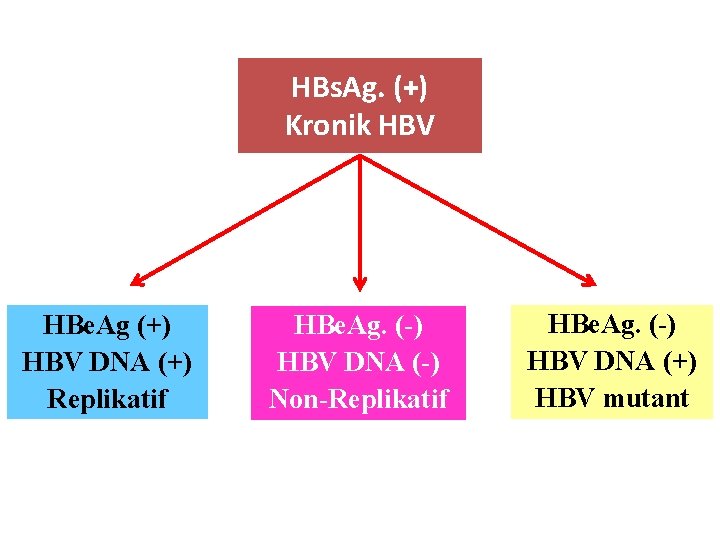
HBs. Ag. (+) Kronik HBV HBe. Ag (+) HBV DNA (+) Replikatif HBe. Ag. (-) HBV DNA (-) Non-Replikatif HBe. Ag. (-) HBV DNA (+) HBV mutant

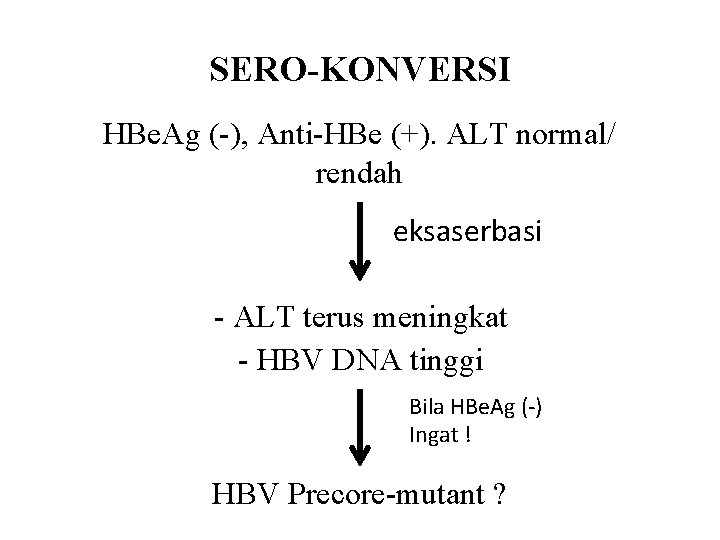
SERO-KONVERSI HBe. Ag (-), Anti-HBe (+). ALT normal/ rendah eksaserbasi - ALT terus meningkat - HBV DNA tinggi Bila HBe. Ag (-) Ingat ! HBV Precore-mutant ?

HBV S gene mutant and diagnostic assays Presence of HBV- DNA in : • Individuals with isolated anti-HBc reactivity* • Patient with discordant result between HBs. Ag assays • Samples with have no no markers of HBV infection* • Patients seronegative for HBs. Ag negative but HBe. Ag positive* • Presence of both HBs. Ag and anti-HBs ( mostly at lower titer < 100 m. UI/m. L) • Individual with isolated anti-HBs reactivity* (without immunization ) *Occult HBV infection 38

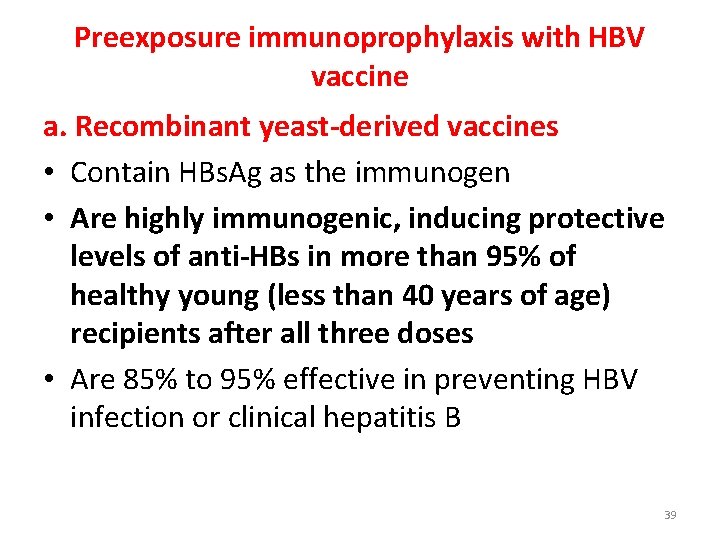
Preexposure immunoprophylaxis with HBV vaccine a. Recombinant yeast-derived vaccines • Contain HBs. Ag as the immunogen • Are highly immunogenic, inducing protective levels of anti-HBs in more than 95% of healthy young (less than 40 years of age) recipients after all three doses • Are 85% to 95% effective in preventing HBV infection or clinical hepatitis B 39

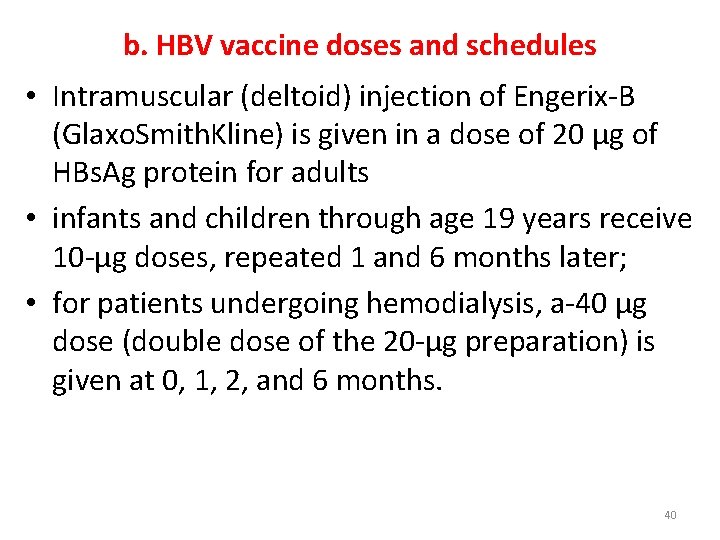
b. HBV vaccine doses and schedules • Intramuscular (deltoid) injection of Engerix-B (Glaxo. Smith. Kline) is given in a dose of 20 μg of HBs. Ag protein for adults • infants and children through age 19 years receive 10 -μg doses, repeated 1 and 6 months later; • for patients undergoing hemodialysis, a-40 μg dose (double dose of the 20 -μg preparation) is given at 0, 1, 2, and 6 months. 40

Chronic Hepatitis B Treatment Nucleotide analogs Adenovir dipivoxil, has activity against wildtype and lamivudine-resistant HBV Telbivudine Entecavir Tenofovir ( HIV infection ) Emtricitabine & Clevudine 41

TERIMA KASIH ATAS PERHATIAN ANDA

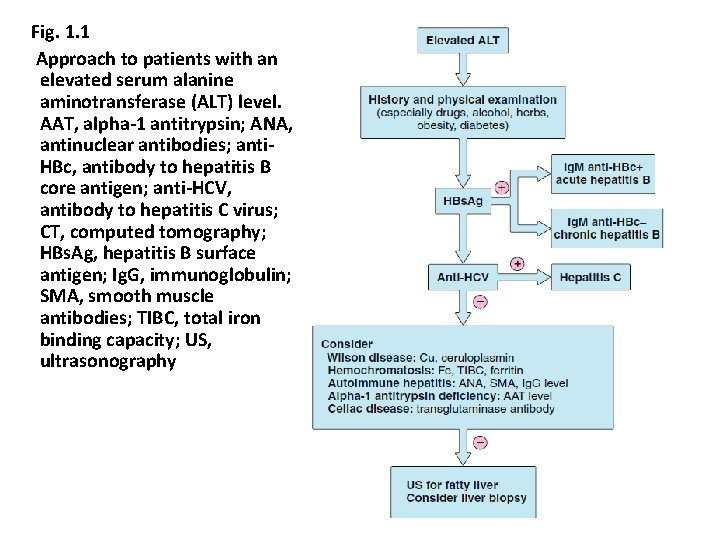
Fig. 1. 1 Approach to patients with an elevated serum alanine aminotransferase (ALT) level. AAT, alpha-1 antitrypsin; ANA, antinuclear antibodies; anti. HBc, antibody to hepatitis B core antigen; anti-HCV, antibody to hepatitis C virus; CT, computed tomography; HBs. Ag, hepatitis B surface antigen; Ig. G, immunoglobulin; SMA, smooth muscle antibodies; TIBC, total iron binding capacity; US, ultrasonography 43

Drugs a. Current first line therapies • Peginterferon alfa-2 a (exceptions: pregnancy, chemotherapy prophylaxis, decompensated cirrhosis) • Entecavir • Tenofovir b. The first decision is selection of either a nucleos(t)ide analogue or peginterferon. for 48 weeks. 44

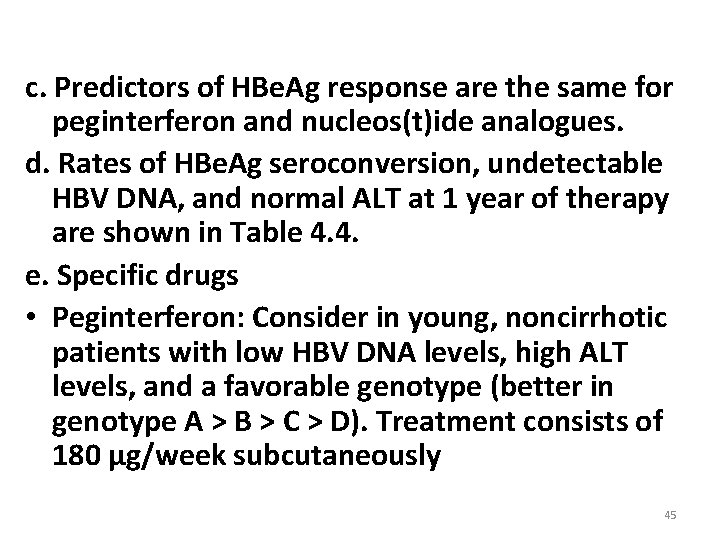
c. Predictors of HBe. Ag response are the same for peginterferon and nucleos(t)ide analogues. d. Rates of HBe. Ag seroconversion, undetectable HBV DNA, and normal ALT at 1 year of therapy are shown in Table 4. 4. e. Specific drugs • Peginterferon: Consider in young, noncirrhotic patients with low HBV DNA levels, high ALT levels, and a favorable genotype (better in genotype A > B > C > D). Treatment consists of 180 μg/week subcutaneously 45

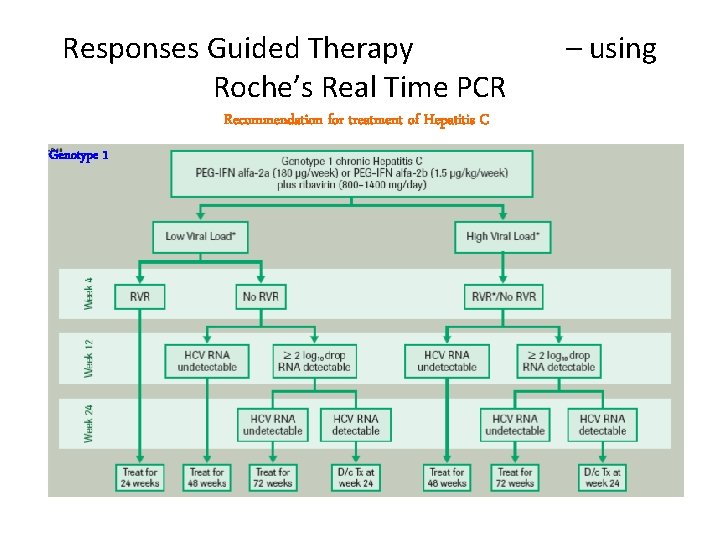
Responses Guided Therapy Roche’s Real Time PCR Recommendation for treatment of Hepatitis C Genotype 1 – using

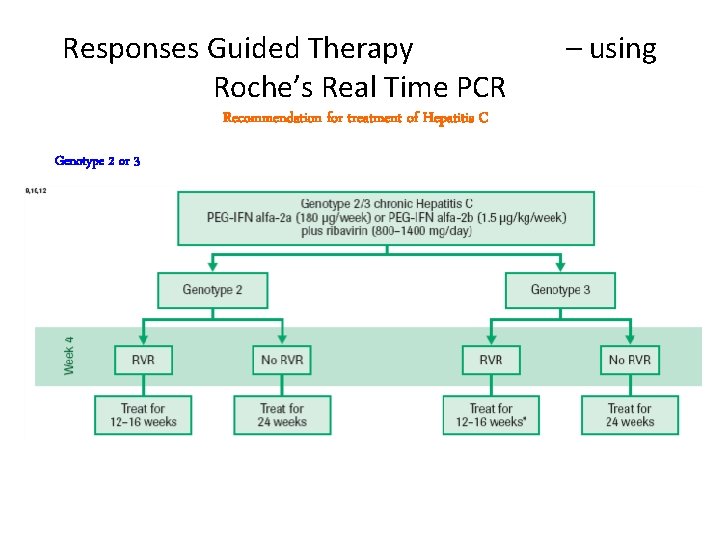
Responses Guided Therapy Roche’s Real Time PCR Recommendation for treatment of Hepatitis C Genotype 2 or 3 – using

• Have principal side effects of – transient pain at the injection site in 10% to 25% – short-lived, mild fever in less than 3% • Should not have boosters even as long as 20 years after initial immunization (may provide lifelong protection) • Should have boosters only for immunocompromised individuals if the anti-HBs titer is less than 10 m. U/m. L on annual testing • Have no proved immunotherapeutic value in the individual with established HBV infection 48

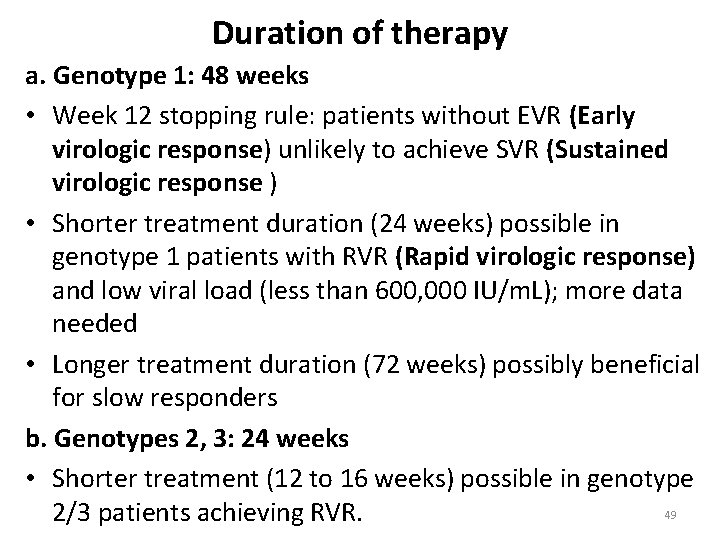
Duration of therapy a. Genotype 1: 48 weeks • Week 12 stopping rule: patients without EVR (Early virologic response) unlikely to achieve SVR (Sustained virologic response ) • Shorter treatment duration (24 weeks) possible in genotype 1 patients with RVR (Rapid virologic response) and low viral load (less than 600, 000 IU/m. L); more data needed • Longer treatment duration (72 weeks) possibly beneficial for slow responders b. Genotypes 2, 3: 24 weeks • Shorter treatment (12 to 16 weeks) possible in genotype 49 2/3 patients achieving RVR.

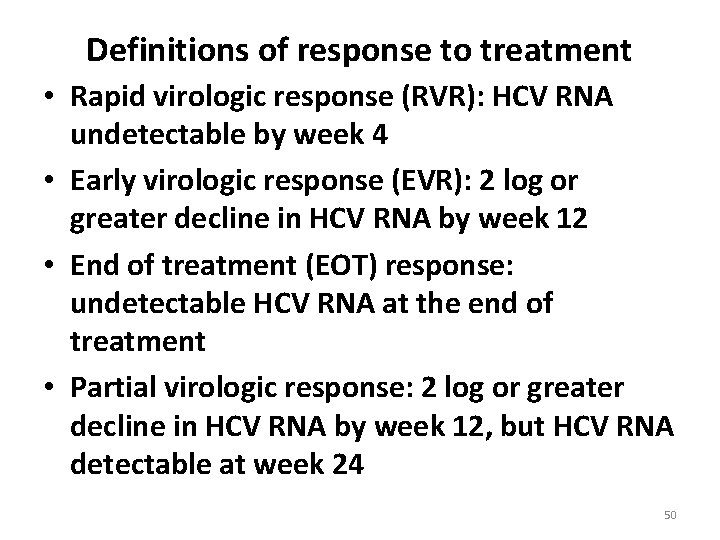
Definitions of response to treatment • Rapid virologic response (RVR): HCV RNA undetectable by week 4 • Early virologic response (EVR): 2 log or greater decline in HCV RNA by week 12 • End of treatment (EOT) response: undetectable HCV RNA at the end of treatment • Partial virologic response: 2 log or greater decline in HCV RNA by week 12, but HCV RNA detectable at week 24 50

• Sustained virologic response (SVR): HCV RNA negativity 12 to 24 weeks after the end of treatment 51

CHRONIC VIRAL HEPATITIS ESSENTIAL OF DIAGNOSIS ▪ Defined by chronic infection (HBV, HCV, HDV) for > 6 months ▪ Diagnosis is usually made by antibody tests and viral nucleic acid in serum CMTD, 2011 52

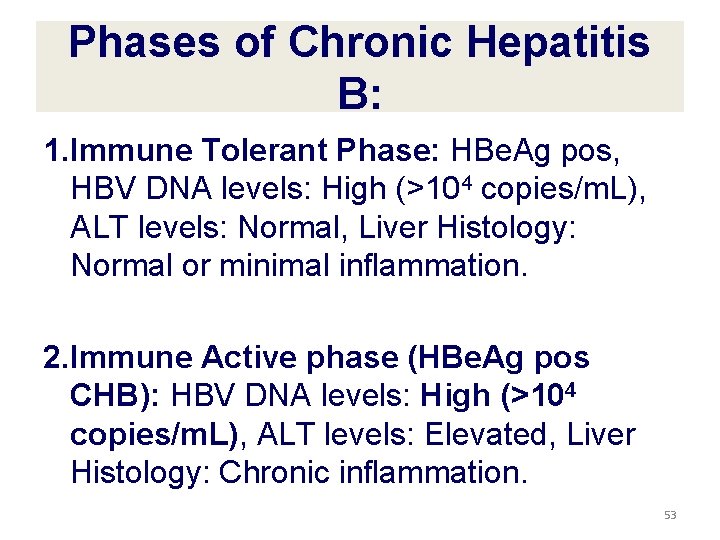
Phases of Chronic Hepatitis B: 1. Immune Tolerant Phase: HBe. Ag pos, HBV DNA levels: High (>104 copies/m. L), ALT levels: Normal, Liver Histology: Normal or minimal inflammation. 2. Immune Active phase (HBe. Ag pos CHB): HBV DNA levels: High (>104 copies/m. L), ALT levels: Elevated, Liver Histology: Chronic inflammation. 53