Pediatric Immunizations Part 1 Prepared by Dr Latifa

Pediatric Immunizations Part 1 Prepared by : Dr. Latifa Mari’e

A moment’s cry in the name of prevention Pediatric Immunizations
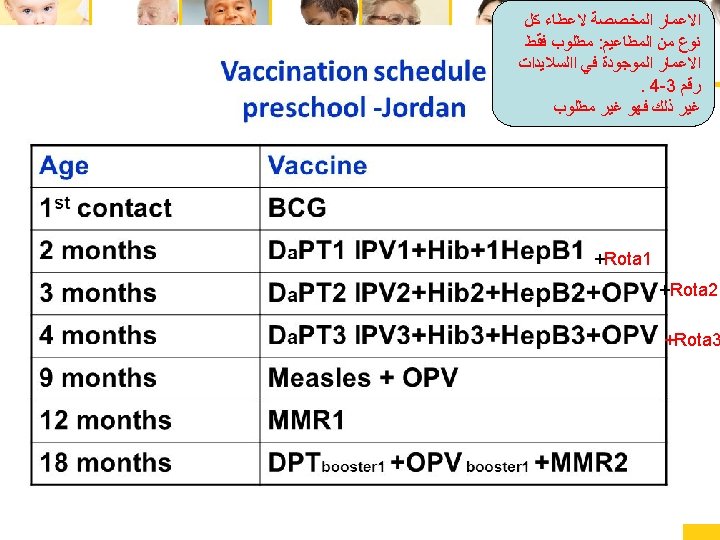

• 1 st +10 th grade (td and opv for 1 st class) Pediatric Immunizations

Name some vaccine preventable diseases Pediatric Immunizations

Vaccine Preventable Diseases • Tetanus • Diphtheria • Pertussis (Whooping Cough) • Hemophilis influenza type b (Hib) • Polio • Hepatitis A • Hepatitis B • Rotavirus • • Mumps Measles Rubella (German Measles) Varicella (Chickenpox) Pneumococcus Meningococcus Influenza Human Papilloma Virus (HPV) Pediatric Immunizations

Vaccine Preventable Diseases • Herd immunity plus aggressive immunization has made most of these diseases rare • Most current residents and medical students haven’t seen these diseases Pediatric Immunizations

Herd Immunity • Most of population immunized • Disease itself still exists, but spread prevented by lack of available hosts • Unimmunized person less likely to come in contact with infected person Pediatric Immunizations

Vaccine Preventable Diseases • To comprehend why we immunize, you – Need to have basic childhood disease knowledge – Need to know the combinations of vaccines available to prevent the illnesses Pediatric Immunizations
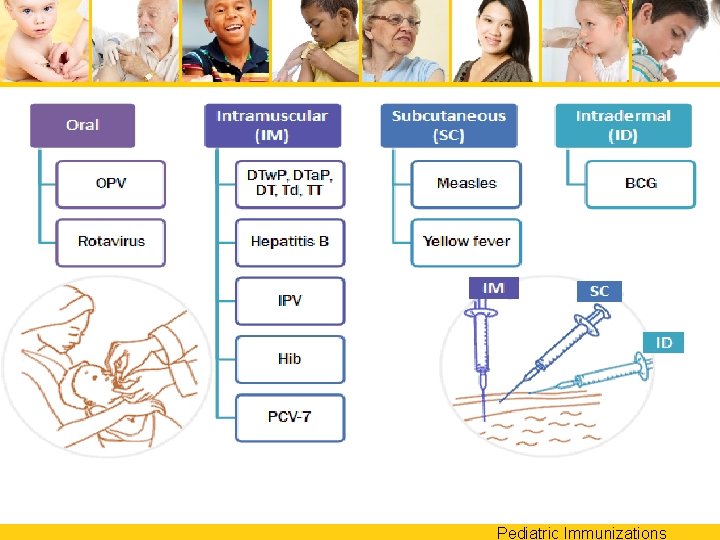
Pediatric Immunizations
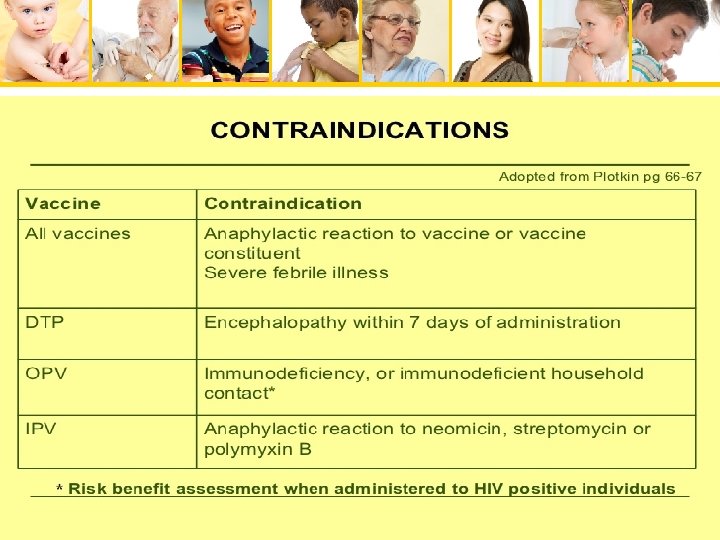
Pediatric Immunizations

Pediatric Immunizations

Bacillus Calmette– Guérin (BCG) vaccine is a vaccine primarily used against tuberculosis (TB). In countries where tuberculosis or leprosy is common, one dose is recommended in healthy babies as close to the time of birth as possible Rates of protection against tuberculosis infection vary widely and protection lasts up to twenty years

• BCG is given as a single intradermal injection at the insertion of the deltoid • BCG vaccine may cause side effects: • swollen lymph nodes. • small red areas at the site of injection. • fever. • blood in the urine. • frequent or painful urination. • upset stomach. • vomiting. Pediatric Immunizations

• BCG complications can cause significant morbidity in children and anxiety in their parents. Local adverse reactions include regional suppurative and nonsuppurative lymphadenitis, injection site abscesses, persistent injection site reactions, ulceration and uncommonly keloid reactions Pediatric Immunizations
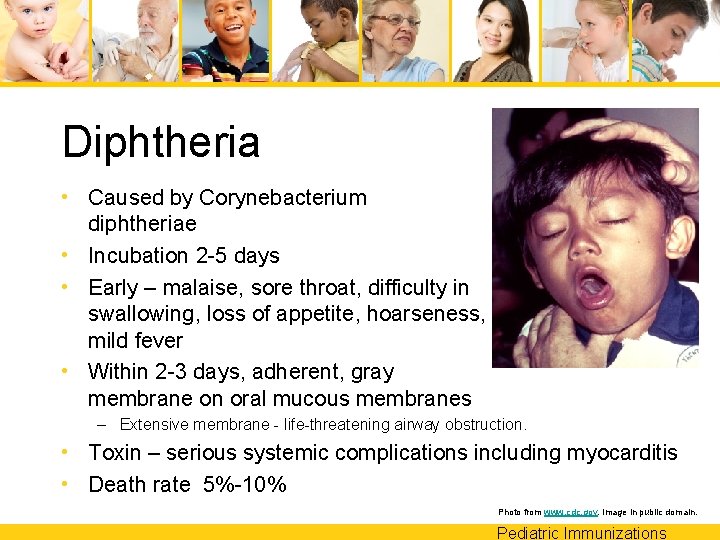
Diphtheria • Caused by Corynebacterium diphtheriae • Incubation 2 -5 days • Early – malaise, sore throat, difficulty in swallowing, loss of appetite, hoarseness, mild fever • Within 2 -3 days, adherent, gray membrane on oral mucous membranes – Extensive membrane - life-threatening airway obstruction. • Toxin – serious systemic complications including myocarditis • Death rate 5%-10% Photo from www. cdc. gov. Image in public domain. Pediatric Immunizations

Tetanus • Spread by contact with soil containing bacterium Clostridium tetani • Most infections from contaminated wounds • Incubation 1 -2 weeks • Not contagious • Produces exotoxin Pediatric Immunizations

Tetanus • 1 -2 weeks after infection – progressive muscle tightening, descending pattern – – Trismus (lockjaw) Neck stiffness Difficulty swallowing Abdominal muscle rigidity • Neonatal tetanus due to no maternal immunity and cutting the umbilical cord with a contaminated instrument (e. g. bamboo in Haiti) Pediatric Immunizations

Tetanus Neonatal tetanus Child with painful muscle contractions from tetanus Photos from www. cdc. gov. Images in public domain. This patient is displaying a bodily posture known as “opisthotonos” due to tetanus. Pediatric Immunizations
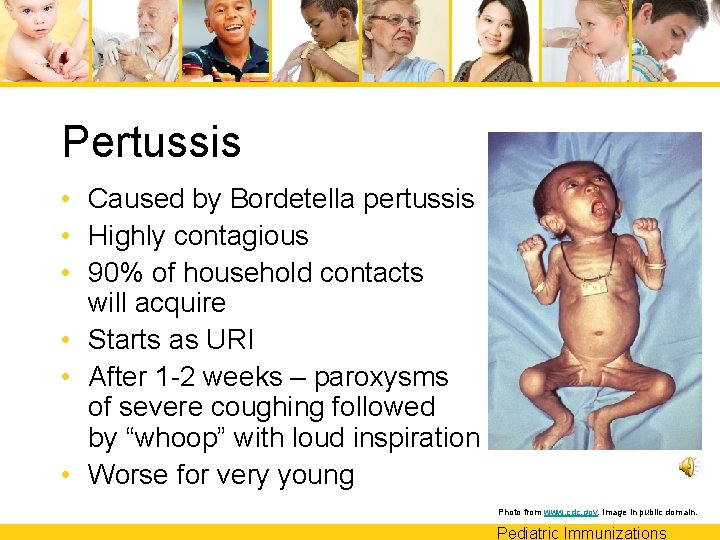
Pertussis • Caused by Bordetella pertussis • Highly contagious • 90% of household contacts will acquire • Starts as URI • After 1 -2 weeks – paroxysms of severe coughing followed by “whoop” with loud inspiration • Worse for very young Photo from www. cdc. gov. Image in public domain. Pediatric Immunizations

Pertussis • Symptoms can last several weeks – Severe coughing – Whooping – Post-tussive vomiting • 1 in 10 cases develop pneumonia • 1 in 50 cases develop convulsions • 1 in 250 cases develop encephalopathy Pediatric Immunizations

DTa. P • Capital letter denotes full dose vaccine • Small “a” for acellular • Compared to Td or Tdap – Small letter denotes half dose vaccine for booster effect • Diphtheria and Pertussis vaccines only given as combination with Tetanus Pediatric Immunizations

DTa. P • • Diphtheria Tetanus Acellular pertussis Primary series – – – 2, 4, 6 months 12 -18 months (at least 6 months from the 3 rd dose) 4 years 12 -14 years Tdap Then Td boosters every 10 years Pediatric Immunizations
DTa. P • Contraindications – Severe allergic reaction (e. g. , anaphylaxis) after a previous vaccine dose or to a vaccine component – Encephalopathy (e. g. , coma, decreased level of consciousness; prolonged seizures) • not attributable to another identifiable cause • within 7 days of administration of previous dose of DTP or DTa. P – Progressive neurologic disorder • including infantile spasms • uncontrolled epilepsy • progressive encephalopathy – Defer DTa. P until neurologic status clarified and stabilized Pediatric Immunizations
DTa. P • Precautions – Temperature of >104°F (>40. 5°C) • For <48 hours after a previous dose of DTP or DTa. P – Collapse or shock-like state • Occurs <48 hours after a previous dose of DTP/DTa. P – Seizure • <3 days after a previous dose of DTP/DTa. P – Persistent, inconsolable crying • lasting >3 hours within 48 hours of a dose of DTP/DTa. P – Guillain-Barre syndrome (GBS) • <6 weeks after dose of tetanus toxoid-containing vaccine – Moderate or severe acute illness with or without fever Pediatric Immunizations

Safe Situations to Administer DTa. P • • Temperature of <105°F (<40. 5°C) after dose Fussiness after dose Mild drowsiness after dose Family history of seizures Family history of sudden infant death syndrome Family history of an adverse event after vaccine Stable neurologic conditions – cerebral palsy – well-controlled seizure disorder – developmental delay Pediatric Immunizations
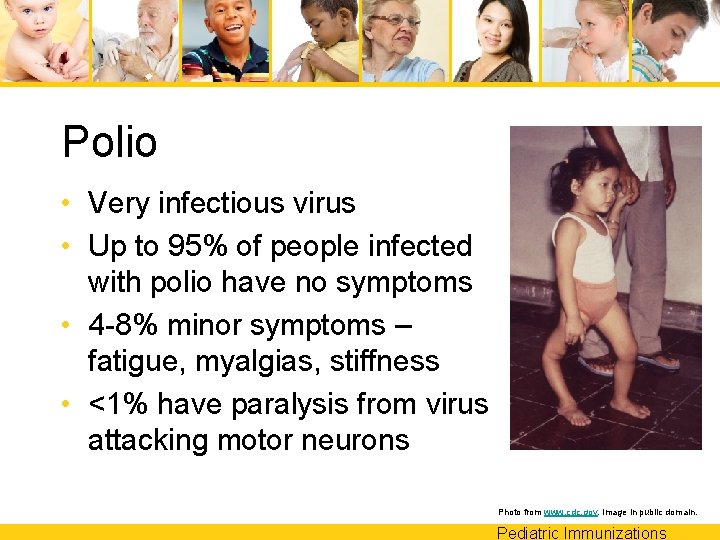
Polio • Very infectious virus • Up to 95% of people infected with polio have no symptoms • 4 -8% minor symptoms – fatigue, myalgias, stiffness • <1% have paralysis from virus attacking motor neurons Photo from www. cdc. gov. Image in public domain. Pediatric Immunizations

IPV • Inactivated polio vaccine • 4 dose series – 2, 4, 6 -18 months – Booster at 4 years • Dose 4 must be 6 months after 3 rd dose • If dose 3 is after 4 years old and >6 months from dose 2, a 4 th dose is not needed • If 4 doses received prior to 4 years old, a 5 th dose is required Pediatric Immunizations

contraindications • Previous anaphylactic rxn • Precautions • Moderate to severe acute illness • pregnancy Pediatric Immunizations
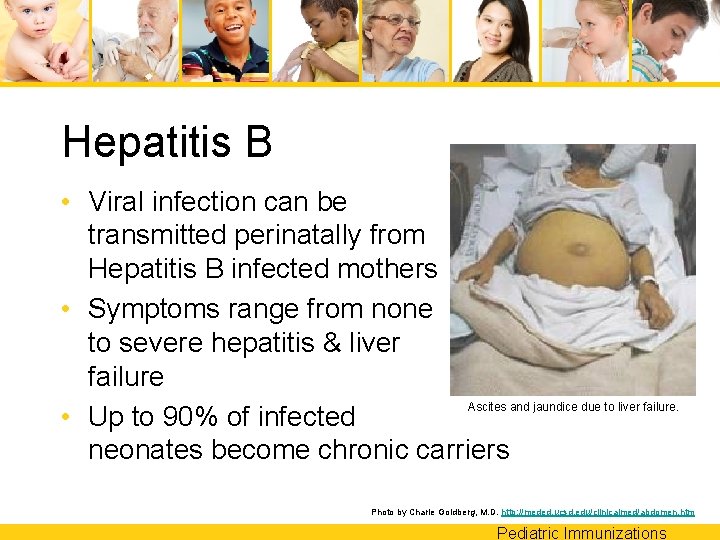
Hepatitis B • Viral infection can be transmitted perinatally from Hepatitis B infected mothers • Symptoms range from none to severe hepatitis & liver failure Ascites and jaundice due to liver failure. • Up to 90% of infected neonates become chronic carriers Photo by Charle Goldberg, M. D. http: //meded. ucsd. edu/clinicalmed/abdomen. htm Pediatric Immunizations

Engerix-B® (HBV) • Recombinant Hepatitis B • 3 doses, given IM • Dose should be given shortly after birth – If not given to infant • Document your order to hold vaccine • Copy of negative maternal Hepatitis B surface antigen (Hbs. Ag) in chart and documented by physician • If mom HBs. Ag positive – Give HBV and Hepatitis B immunoglobulin (HBIG) within 12 hours of birth – Test for Hbs. Ag and Hbs. Ab 1 -2 months after completed vaccine series at age 9 -18 months Pediatric Immunizations

Contraindications • Previous anaphylactic rxn • Precautions • Moderate to severe acute illness Pediatric Immunizations
Hemophilus Influenza B (Hib) • Prior to vaccine, Hib was leading cause of childhood – Bacterial meningitis – Epiglottitis – Pneumonia – Empyema – Pericarditis – Bacteremia – Septic arthritis Photo courtesy of Children’s Immunization Project, St. Paul, Minnesota Pediatric Immunizations

ﻏﻴﺮ ﻣﻄﻠﻮﺏ Hemophilus Influenza B (Hib) • 2 vaccines available – 1 is 3 -dose series (Pedvax. HIB®) – 1 is 4 -dose series (Act. HIB®) • Vaccines are interchangeable – If changed at 2 or 4 months of age, need a 6 -month dose of either vaccine – Either vaccine may be given for the 12 -month booster dose Pediatric Immunizations

Hemophilus Influenza B (Hib) • Cannot give any form of Hib to infants less than 6 weeks old – Have decreased immune response to polysaccharide capsule (PRP) of Hib • May also prevent future ability to develop antibodies Pediatric Immunizations

Case Tanya exclaims, “Those are scary diseases! That’s a lot of shots!” Do any vaccines come in combination? YES! Pediatric Immunizations

ﻏﻴﺮ ﻣﻄﻠﻮﺏ Combination Vaccines • Many vaccines come in combinations • However, different manufacturers make different components – Multiple different combinations – Vaccination schedule may vary based on which vaccines given or available Pediatric Immunizations
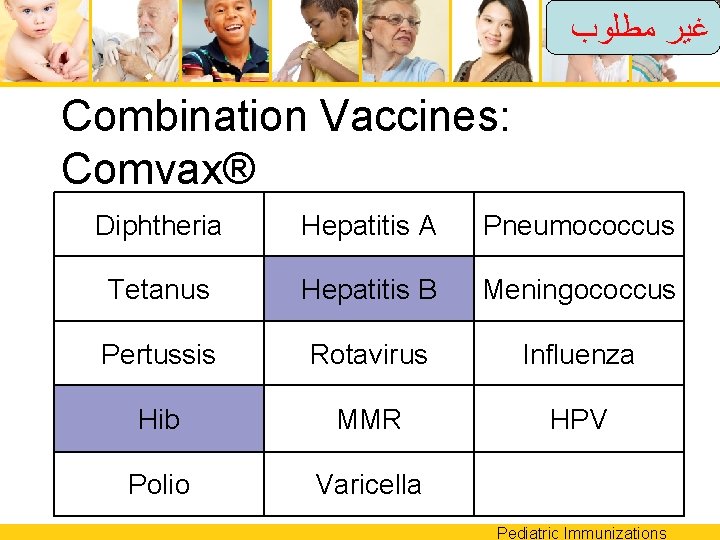
ﻏﻴﺮ ﻣﻄﻠﻮﺏ Combination Vaccines: Comvax® Diphtheria Hepatitis A Pneumococcus Tetanus Hepatitis B Meningococcus Pertussis Rotavirus Influenza Hib MMR HPV Polio Varicella Pediatric Immunizations
ﻏﻴﺮ ﻣﻄﻠﻮﺏ Combination Vaccines: Comvax® • Hepatitis B + Hib (Pedvax. HIB®) • Remember – cannot give Hib before 6 weeks of age – Cannot use Comvax® as Hepatitis B birth or 1 month dose • Doses at 2, 4, >12 months – Final dose after age 12 months – Interchangable with Pedvax. HIB® Pediatric Immunizations
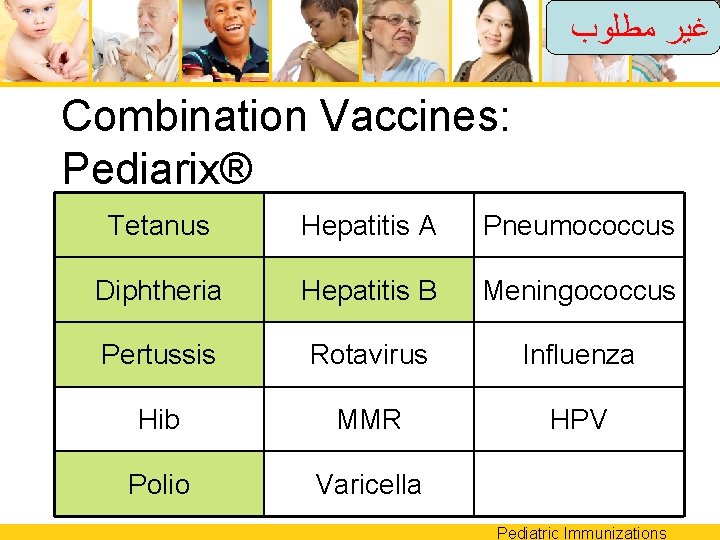
ﻏﻴﺮ ﻣﻄﻠﻮﺏ Combination Vaccines: Pediarix® Tetanus Hepatitis A Pneumococcus Diphtheria Hepatitis B Meningococcus Pertussis Rotavirus Influenza Hib MMR HPV Polio Varicella Pediatric Immunizations
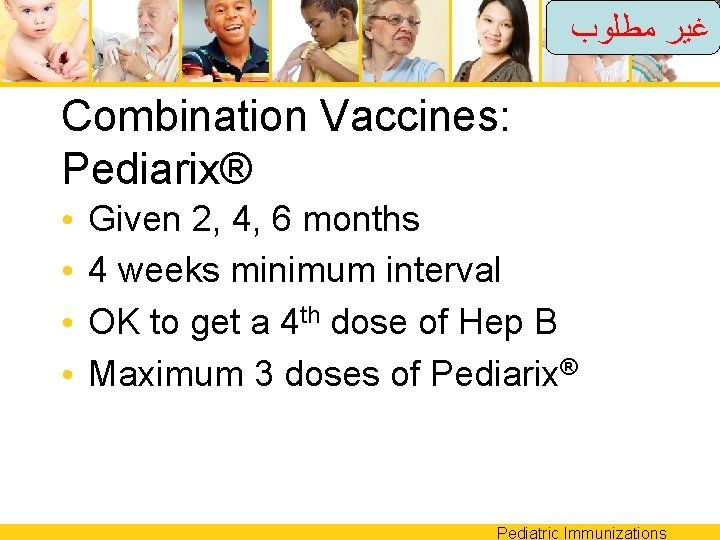
ﻏﻴﺮ ﻣﻄﻠﻮﺏ Combination Vaccines: Pediarix® • • Given 2, 4, 6 months 4 weeks minimum interval OK to get a 4 th dose of Hep B Maximum 3 doses of Pediarix® Pediatric Immunizations
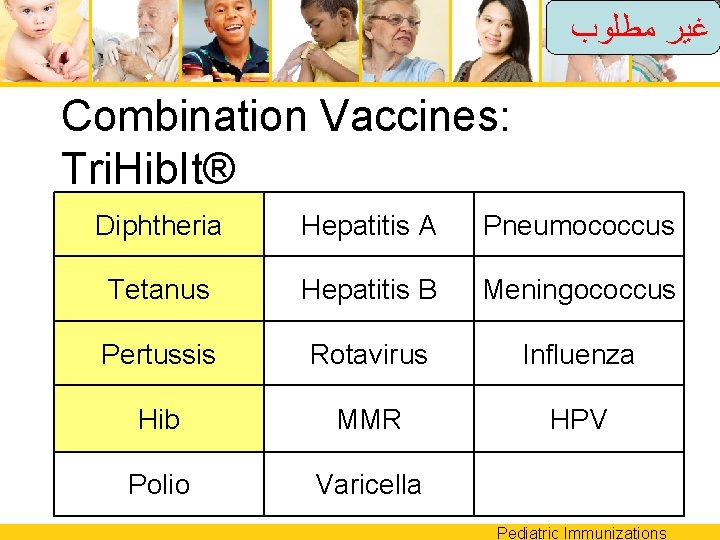
ﻏﻴﺮ ﻣﻄﻠﻮﺏ Combination Vaccines: Tri. Hib. It® Diphtheria Hepatitis A Pneumococcus Tetanus Hepatitis B Meningococcus Pertussis Rotavirus Influenza Hib MMR HPV Polio Varicella Pediatric Immunizations
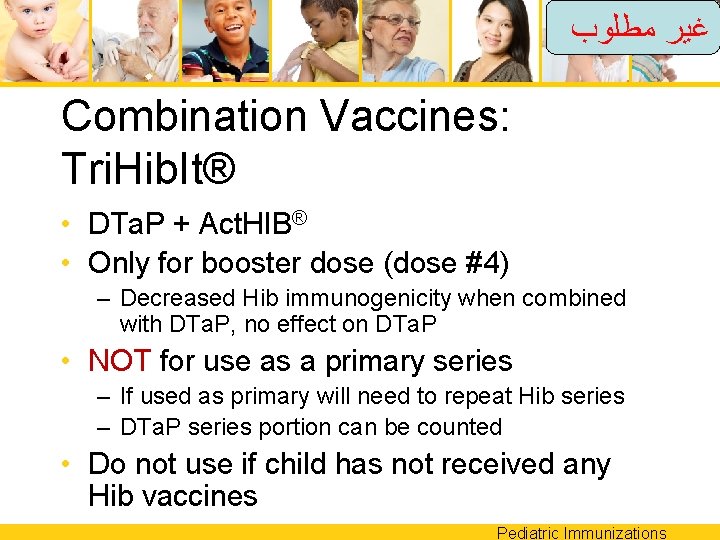
ﻏﻴﺮ ﻣﻄﻠﻮﺏ Combination Vaccines: Tri. Hib. It® • DTa. P + Act. HIB® • Only for booster dose (dose #4) – Decreased Hib immunogenicity when combined with DTa. P, no effect on DTa. P • NOT for use as a primary series – If used as primary will need to repeat Hib series – DTa. P series portion can be counted • Do not use if child has not received any Hib vaccines Pediatric Immunizations
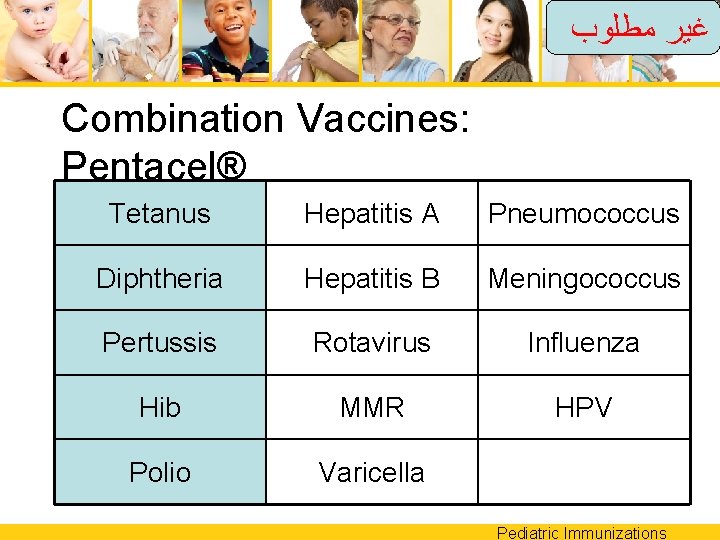
ﻏﻴﺮ ﻣﻄﻠﻮﺏ Combination Vaccines: Pentacel® Tetanus Hepatitis A Pneumococcus Diphtheria Hepatitis B Meningococcus Pertussis Rotavirus Influenza Hib MMR HPV Polio Varicella Pediatric Immunizations
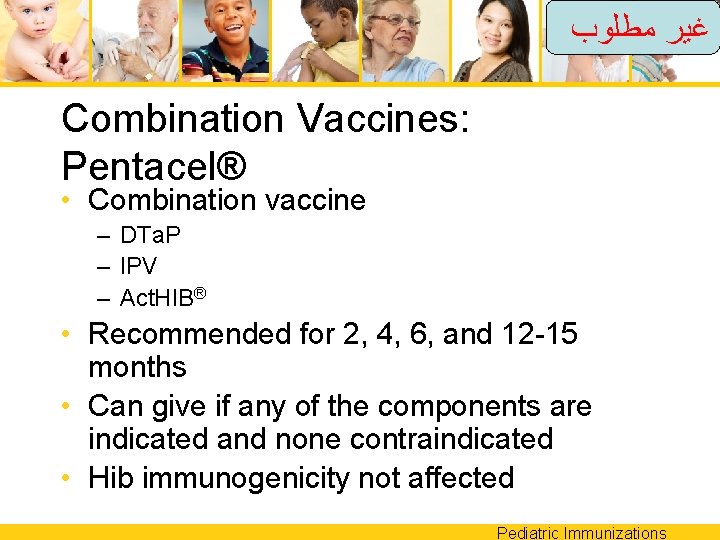
ﻏﻴﺮ ﻣﻄﻠﻮﺏ Combination Vaccines: Pentacel® • Combination vaccine – DTa. P – IPV – Act. HIB® • Recommended for 2, 4, 6, and 12 -15 months • Can give if any of the components are indicated and none contraindicated • Hib immunogenicity not affected Pediatric Immunizations

note • Hib vaccine is not routinely giver to children age 5 yrs and older Pediatric Immunizations

contraindications • Anaphylactic rxn • Age <6 weeks • Precautions • Acute moderate to severe illness Pediatric Immunizations

Case Tanya ponders, “Wasn’t there some vaccine that was going to stop my baby from having ear infections? Which one is that? ” Pediatric Immunizations

Pneumococcal Disease • Streptococcus pneumoniae – Common cause of community acquired pneumonia and otitis media • Can cause invasive disease – Bacteremia – Meningitis – Sepsis pneumococcal meningitis at autopsy • Invasive risk related to serotype present Photo from www. cdc. gov. Image in public domain. Pediatric Immunizations

Prevnar® (PCV-7) • Pneumococcal conjugate 7 valent vaccine • 2, 4, 6 and 12 months • Recommended for all children 2 -23 months • Give if 24 -59 months old with risk factors • Not for children >5 years old • Replaced by PCV-13 Spring 2010 Pediatric Immunizations

PCV-7 X PCV-13 (Prevnar™ 13) ACIP voted 2/24/10 to replace PCV-7 Transition guidelines published Protects against 13 instead of 7 strains Expanded vaccination for high-risk groups to 72 months • Same dosing interval as PCV-7 for never vaccinated children • • Pediatric Immunizations

PCV-13 • High risk children include – Immunocompetent children with • • Cyanotic congenital heart defects Chronic lung disease Asthma needing oral steroid treatment Diabetes CSF leaks Cochlear implants Asplenia (congenital or acquired) Sickle cell and other hemoglobinopathies Pediatric Immunizations

PCV-13 • High risk children include – Immunocompromised children • • HIV Chronic renal failure Nephrotic syndrome Lymphoma and leukemia Chemotherapy Organ transplant Congenital immunodeficiencies Pediatric Immunizations
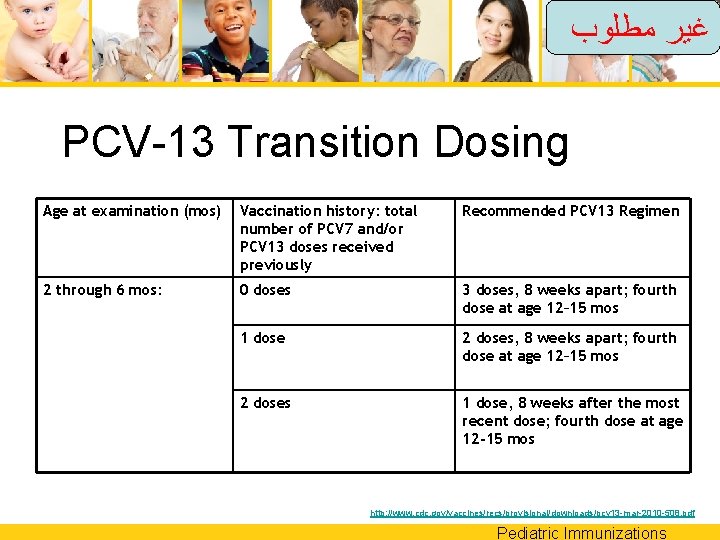
ﻏﻴﺮ ﻣﻄﻠﻮﺏ PCV-13 Transition Dosing Age at examination (mos) Vaccination history: total number of PCV 7 and/or PCV 13 doses received previously Recommended PCV 13 Regimen 2 through 6 mos: 0 doses 3 doses, 8 weeks apart; fourth dose at age 12– 15 mos 1 dose 2 doses, 8 weeks apart; fourth dose at age 12– 15 mos 2 doses 1 dose, 8 weeks after the most recent dose; fourth dose at age 12 -15 mos http: //www. cdc. gov/vaccines/recs/provisional/downloads/pcv 13 -mar-2010 -508. pdf Pediatric Immunizations
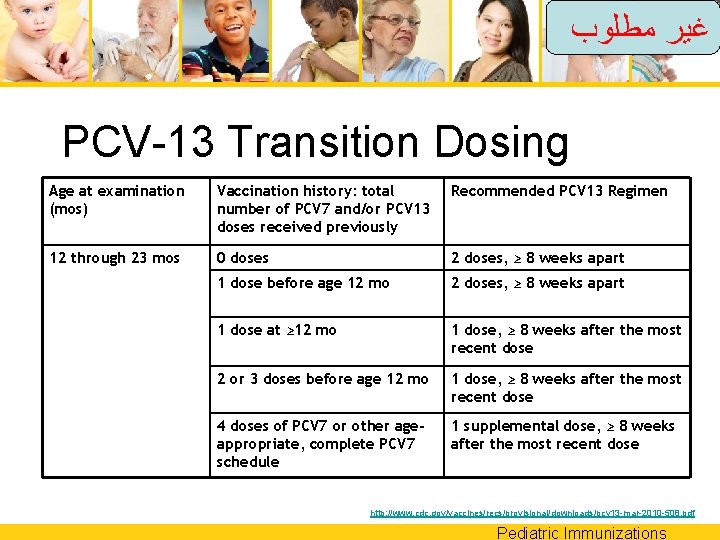
ﻏﻴﺮ ﻣﻄﻠﻮﺏ PCV-13 Transition Dosing Age at examination (mos) Vaccination history: total number of PCV 7 and/or PCV 13 doses received previously Recommended PCV 13 Regimen 12 through 23 mos 0 doses 2 doses, ≥ 8 weeks apart 1 dose before age 12 mo 2 doses, ≥ 8 weeks apart 1 dose at ≥ 12 mo 1 dose, ≥ 8 weeks after the most recent dose 2 or 3 doses before age 12 mo 1 dose, ≥ 8 weeks after the most recent dose 4 doses of PCV 7 or other ageappropriate, complete PCV 7 schedule 1 supplemental dose, ≥ 8 weeks after the most recent dose http: //www. cdc. gov/vaccines/recs/provisional/downloads/pcv 13 -mar-2010 -508. pdf Pediatric Immunizations
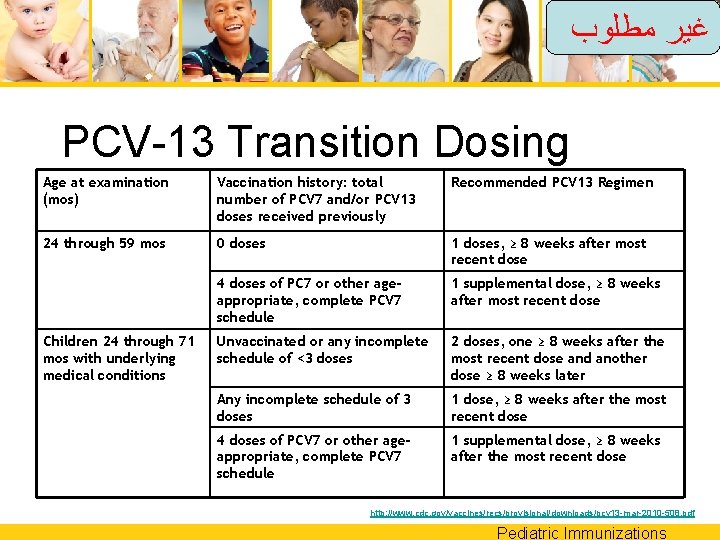
ﻏﻴﺮ ﻣﻄﻠﻮﺏ PCV-13 Transition Dosing Age at examination (mos) Vaccination history: total number of PCV 7 and/or PCV 13 doses received previously Recommended PCV 13 Regimen 24 through 59 mos 0 doses 1 doses, ≥ 8 weeks after most recent dose 4 doses of PC 7 or other ageappropriate, complete PCV 7 schedule 1 supplemental dose, ≥ 8 weeks after most recent dose Unvaccinated or any incomplete schedule of <3 doses 2 doses, one ≥ 8 weeks after the most recent dose and another dose ≥ 8 weeks later Any incomplete schedule of 3 doses 1 dose, ≥ 8 weeks after the most recent dose 4 doses of PCV 7 or other ageappropriate, complete PCV 7 schedule 1 supplemental dose, ≥ 8 weeks after the most recent dose Children 24 through 71 mos with underlying medical conditions http: //www. cdc. gov/vaccines/recs/provisional/downloads/pcv 13 -mar-2010 -508. pdf Pediatric Immunizations

New for PCV-13 • Single dose for children 6 -18 years old at increased risk for invasive pneumococcal disease • Give regardless of previous PCV-7 or PPSV-23 vaccination • Includes: – – Sickle cell disease HIV (or other immunocompromised state) Cochlear implant CSF leaks Pediatric Immunizations

contraindications • Anaphylactic rxn • Precautions • Acute moderate to severe illness Pediatric Immunizations

Case You advise Tanya: Ear infections are caused by a lot of different bacteria and viruses, so some ear infections will be prevented by the pneumococcal vaccine, but not all. PCV-13 is more than 90% effective against invasive disease (the really severe infections). Pediatric Immunizations

Case Tanya asks, “Are all the vaccines shots? I thought some were given orally. ” Pediatric Immunizations

Oral Vaccines • Oral Polio no longer available in the U. S. • Rotavirus vaccine is given orally Pediatric Immunizations

Rotavirus • Most common cause of gastroenteritis worldwide • Can cause severe dehydration • Prior to vaccine introduction in 2006 – 80% of U. S. children had disease by age 5 – 410, 000 physician visits – 205, 000 to 272, 000 ER visits – 55, 000 to 70, 000 hospitalizations – $1 Billion direct/indirect costs Pediatric Immunizations

Rotavirus Vaccine • Contraindications – Severe allergic reaction after a previous dose or to a vaccine component • Precautions – Moderate or severe acute illness with or without fever – Immunosuppression – Receipt of an antibody-containing blood product within 6 weeks – Pre-existing gastrointestinal disease – Previous history of intussusception Pediatric Immunizations

Rotavirus Vaccine • First vaccine was Rotashield® (RRV-TV) • Licensed 1998 • Withdrawn from market less than 1 year later due to increased risk of intussusception • New Rotavirus vaccines do not show increased risk for intussusception Pediatric Immunizations

Rotavirus Vaccine • 2 currently available are interchangeable for dosing – Rota. Teq® (RV 5) – Rotarix® (RV 1) • Narrow administration window – First dose must be before 15 weeks – Last dose must be before 8 months Pediatric Immunizations

Rotavirus Efficacy and Safety Trial (REST) • 4, 512 infants through 1 st rotavirus season after vaccination with RV 5 Vaccine (Rota. Teq®) • Reduced severe rotavirus gastroenteritis cases by 98% – In other trials – RV 1 vaccine (Rotarix®) reduced by 96% • Reduced any severity case of rotavirus by 74% – RV 1 vaccine reduced by 86% Pediatric Immunizations

Rotavirus Efficacy and Safety Trial (REST) • RV 5 vaccination reduced health care utilization – Reduced ER visits by 93% – Reduced office visits by 86% – Reduced hospitalization rates by 95% – Reduced hospitalization rates for all strains rotavirus gastroenteritis by 58% Pediatric Immunizations
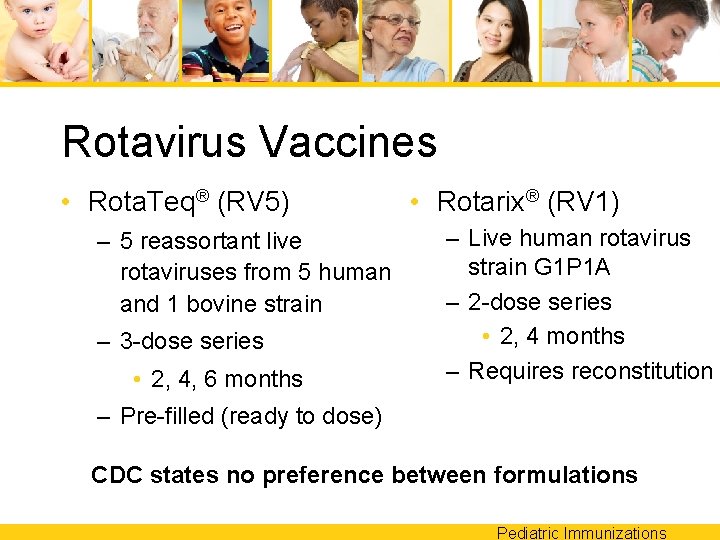
Rotavirus Vaccines • Rota. Teq® (RV 5) – 5 reassortant live rotaviruses from 5 human and 1 bovine strain – 3 -dose series • 2, 4, 6 months • Rotarix® (RV 1) – Live human rotavirus strain G 1 P 1 A – 2 -dose series • 2, 4 months – Requires reconstitution – Pre-filled (ready to dose) CDC states no preference between formulations Pediatric Immunizations

Case • Tanya’s 2 -month-old child can receive the following regimens: – Pediarix® (Hep B, DTa. P, IPV), Hib, PCV 13, Rotavirus • 3 shots, one oral – Pentacel® (DTa. P, IPV, Hib), Hep B, PCV 13, Rotavirus • 3 shots, one oral – Comvax® (Hep B, Hib), DTa. P, IPV, PCV 13, Rotavirus • 4 shots, one oral Pediatric Immunizations

Case Tanya is very thankful for all your help explaining the vaccines her infant needs. She asks, though, “I have a friend who said I shouldn’t let my baby get any vaccines because they will make her autistic. Is that really true? ” Pediatric Immunizations

Case What is your response to Tanya? Parents often express concerns about the thimerosal preservative in vaccines and the MMR vaccine. Healthcare providers should reassure parents. Pediatric Immunizations

Vaccine Safety Vaccination never been proven associated with autism • In 2010, Lancet retracted the 1998 article – Was initial paper linking MMR with autism • There are multiple web-based sites that have false information regarding vaccine risks Pediatric Immunizations

Case Tanya asks, “What about when my baby gets older? How many more vaccines will she need? Are there other diseases she needs to be protected against? ” Pediatric Immunizations

Case What are the routine recommendations for follow-up vaccines? Pediatric Immunizations

Case The routine recommendations are immunizations at: – 2, 4, 6 and 12 -15 months of age Pediatric Immunizations

Case Are there vaccines the child needs in the future that she is too young to receive now? Pediatric Immunizations

Case YES There additional vaccines needed that cannot be administered until after the baby’s first birthday. We will pick up here in “Pediatric Immunizations Part 2” Pediatric Immunizations

Summary • Medical Knowledge – Many childhood diseases are rare due to immunizations • Diphtheria, tetanus, polio, invasive pneumococcal and meningococcal disease, measles, mumps, rubella – Live vaccines for children are MMR, Varicella and Rotavirus – Vaccines come in multiple combinations which vary by manufacturer Pediatric Immunizations
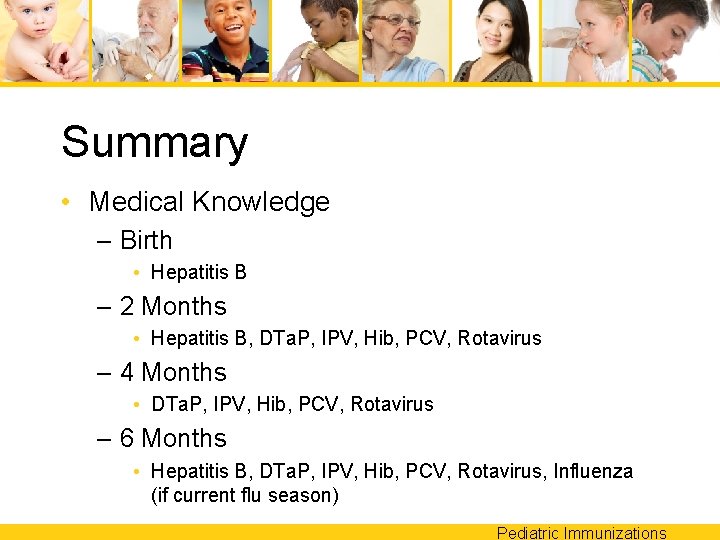
Summary • Medical Knowledge – Birth • Hepatitis B – 2 Months • Hepatitis B, DTa. P, IPV, Hib, PCV, Rotavirus – 4 Months • DTa. P, IPV, Hib, PCV, Rotavirus – 6 Months • Hepatitis B, DTa. P, IPV, Hib, PCV, Rotavirus, Influenza (if current flu season) Pediatric Immunizations

Summary • Patient Care – Birth dose of Hepatitis B must be given (If not given, reason must be well documented) – Do not give Hib vaccine to an infant less than 6 weeks old due to their immature immune system’s inability to produce an appropriate immune response. Pediatric Immunizations

Notes • All live attenuated vaccine must be given on the same day , if not 28 days apart • Preterm immunization is the same for term do not postpone his /her vaccines Pediatric Immunizations

• Thank you Pediatric Immunizations
- Slides: 82