Pediatric Formulation Development A quality perspective Julia C






- Slides: 15

Pediatric Formulation Development A quality perspective Julia C. Pinto, Ph. D. Branch Chief, Office of New Drug Products Office of Product Quality, CDER, FDA

Chemistry, Manufacturing and Controls (CMC) perspective • Extemporaneous preparations using approved adult drug product for pediatric use • Compounding of approved adult drug product for pediatric use • Design and manufacture of drug product for Pediatric patients. www. fda. gov 2

Limitations in Developing Extemporaneous Formulations • Lack of stability/sterility studies • Excipients used for the approved adult formulation • Dosing, Efficacy and safety concerns • Variations in practice www. fda. gov 3

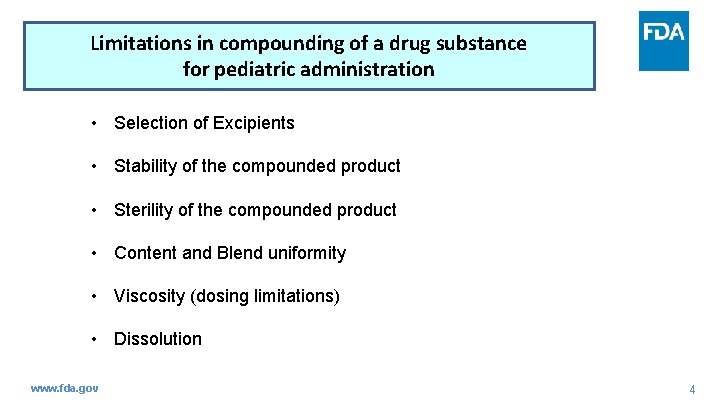
Limitations in compounding of a drug substance for pediatric administration • Selection of Excipients • Stability of the compounded product • Sterility of the compounded product • Content and Blend uniformity • Viscosity (dosing limitations) • Dissolution www. fda. gov 4

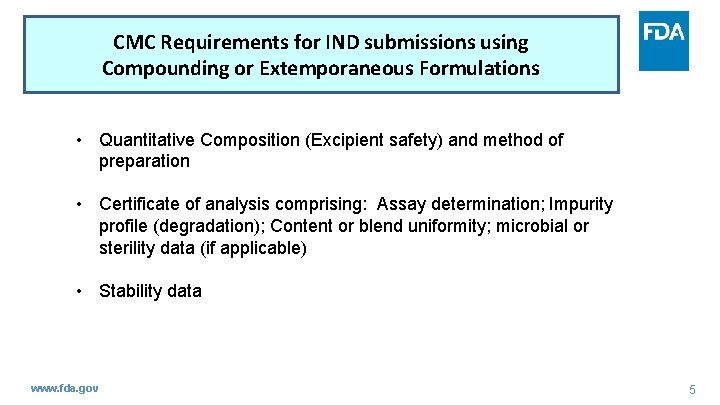
CMC Requirements for IND submissions using Compounding or Extemporaneous Formulations • Quantitative Composition (Excipient safety) and method of preparation • Certificate of analysis comprising: Assay determination; Impurity profile (degradation); Content or blend uniformity; microbial or sterility data (if applicable) • Stability data www. fda. gov 5

Challenges in New Formulation Development • Dose flexibility: accuracy of dosing low doses and small volumes across all the age groups (infants to tweens) • Route of administration and bioequivalence: Inability to swallow tablets/capsules • Patient Compliance: palatability, smell, texture • Choice of Excipients and Toxicity • Physical and Chemical Properties of the Drug Substance Solubility, pka, stability in liquids and foods www. fda. gov 6

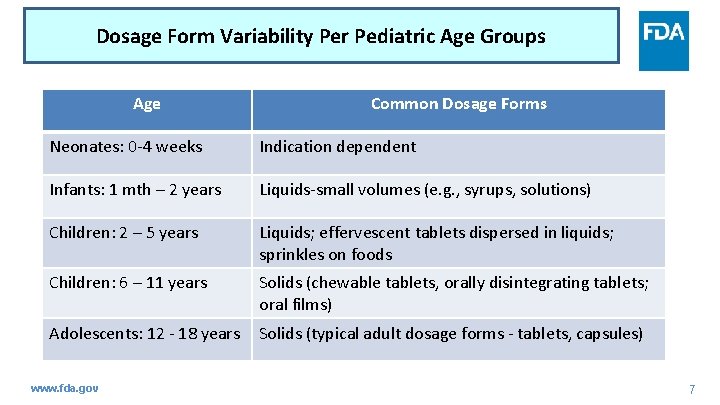
Dosage Form Variability Per Pediatric Age Groups Age Common Dosage Forms Neonates: 0 -4 weeks Indication dependent Infants: 1 mth – 2 years Liquids-small volumes (e. g. , syrups, solutions) Children: 2 – 5 years Liquids; effervescent tablets dispersed in liquids; sprinkles on foods Children: 6 – 11 years Solids (chewable tablets, orally disintegrating tablets; oral films) Adolescents: 12 - 18 years Solids (typical adult dosage forms - tablets, capsules) www. fda. gov 7

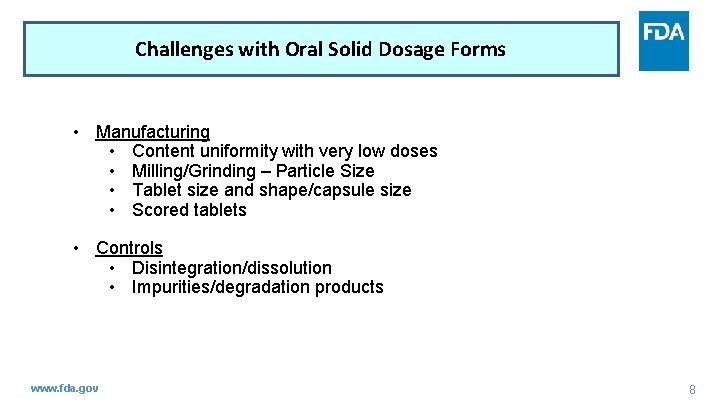
Challenges with Oral Solid Dosage Forms • Manufacturing • Content uniformity with very low doses • Milling/Grinding – Particle Size • Tablet size and shape/capsule size • Scored tablets • Controls • Disintegration/dissolution • Impurities/degradation products www. fda. gov 8

Challenges with Liquid Formulations • Palatability (taste, texture, smell) • Chemical Stability of Ready to Use Solutions or Suspension • Physical Stability of Suspensions • Proper Measuring Device(s) • Suitable Container/Closures (leachable/extractables) • Excipients (safety consideration) www. fda. gov 9

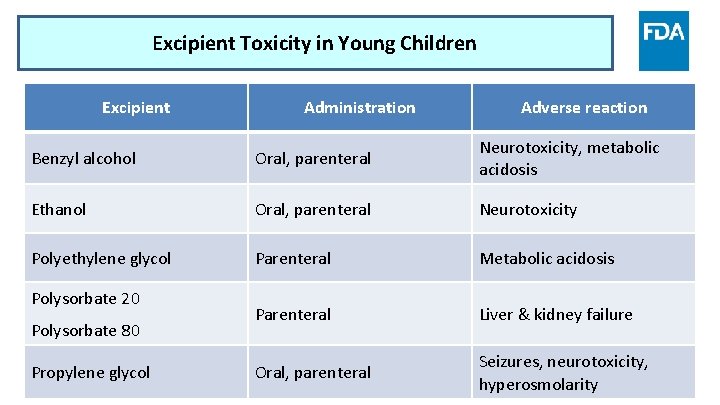
Excipient Toxicity in Young Children Excipient Administration Adverse reaction Benzyl alcohol Oral, parenteral Neurotoxicity, metabolic acidosis Ethanol Oral, parenteral Neurotoxicity Polyethylene glycol Parenteral Metabolic acidosis Parenteral Liver & kidney failure Oral, parenteral Seizures, neurotoxicity, hyperosmolarity Polysorbate 20 Polysorbate 80 Propylene glycol www. fda. gov 10

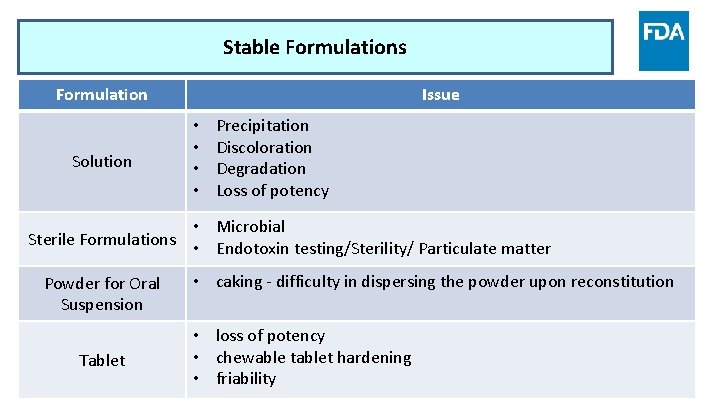
Stable Formulations Formulation Solution Issue • • Precipitation Discoloration Degradation Loss of potency • Microbial Sterile Formulations • Endotoxin testing/Sterility/ Particulate matter Powder for Oral Suspension Tablet www. fda. gov • caking - difficulty in dispersing the powder upon reconstitution • loss of potency • chewable tablet hardening • friability 11

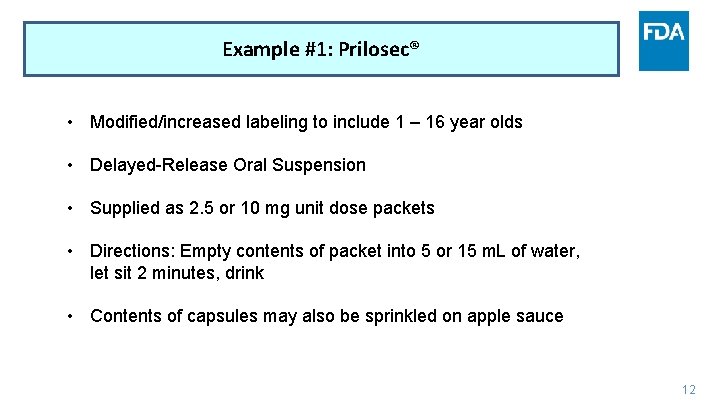
Example #1: Prilosec® • Modified/increased labeling to include 1 – 16 year olds • Delayed-Release Oral Suspension • Supplied as 2. 5 or 10 mg unit dose packets • Directions: Empty contents of packet into 5 or 15 m. L of water, let sit 2 minutes, drink • Contents of capsules may also be sprinkled on apple sauce 12

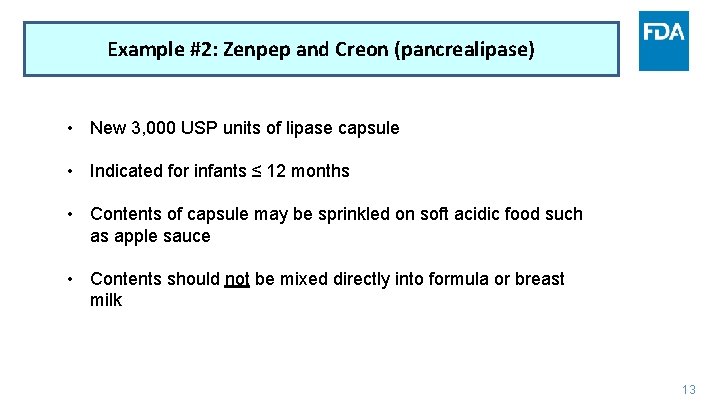
Example #2: Zenpep and Creon (pancrealipase) • New 3, 000 USP units of lipase capsule • Indicated for infants ≤ 12 months • Contents of capsule may be sprinkled on soft acidic food such as apple sauce • Contents should not be mixed directly into formula or breast milk 13

Conclusions: Pediatric Product Design and Manufacture • Critical CMC Controls (compounding/extemporaneous preparations) • API stability (maintain potency) • Degradation (impurity profile) • Blend Uniformity • Dissolution (solid dosage forms) • Microbial/sterility testing • New Product Profile • Dose Flexibility (specific pediatric age group(s)) • Palatable • Stable long term • Maintain Bioequivalence to Adult Formulation 14
