PDP Physics Nuclear fusion and Radioactivity Image http





























- Slides: 66

PDP Physics Nuclear fusion and Radioactivity Image: http: //en. wikipedia. org/wiki/Exoplanet

GENERATING ELECTRICITY

Electricity generation • • • wind hydroelectric coal oil natural gas biofuel solar power geothermal (nuclear)

Electricity generation presentation • in a group of 2 or 3 • on google drive or prezi • about 3 -4 minutes Your presentation should answer the following: • How does it work? • What energy conversions are involved? • How efficient is it? • How much power does a typical plant produce? • How much power is generated in total in Sweden? • Where do the resources originate? • What are the benefits of this method of generating electricity? • What are the problems? How significant are they? • What is the future potential?

Electricity bill in k. Wh http: //www. dolceta. eu/sverige/Mod 6/Elrakningen. html

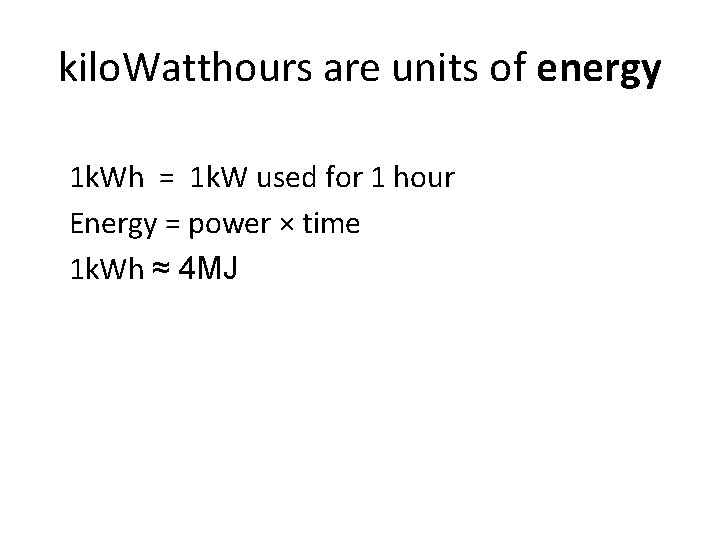
kilo. Watthours are units of energy 1 k. Wh = 1 k. W used for 1 hour Energy = power × time 1 k. Wh ≈ 4 MJ

Comparing different energy uses Fluctuations k. Wh per day k. Wh/d is a unit of power = energy/time

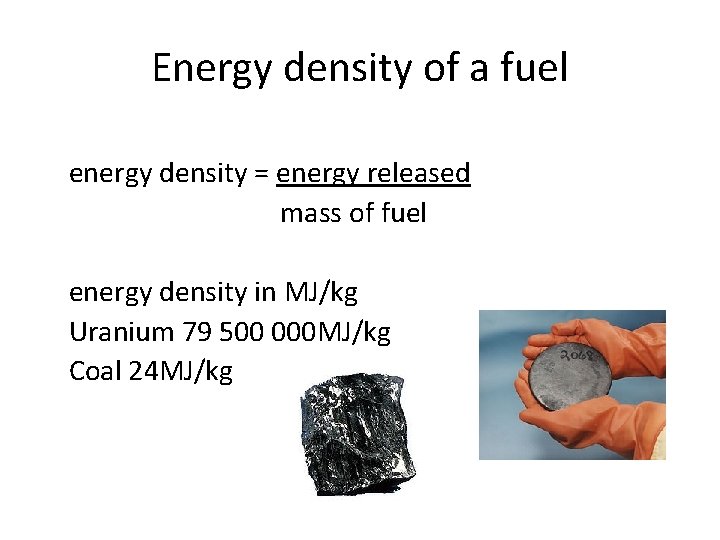
Energy density of a fuel energy density = energy released mass of fuel energy density in MJ/kg Uranium 79 500 000 MJ/kg Coal 24 MJ/kg

Efficiency What percentage of the energy comes out as useful energy? efficiency = energy out × 100 energy in

WHAT IS NUCLEAR FUSION?

WHAT ARE ATOMS BUILT FROM?

Atoms • • atomic number mass number relative atomic mass electron shell isotope atomic mass unit, u elementary charge, e electron. Volt, e. V

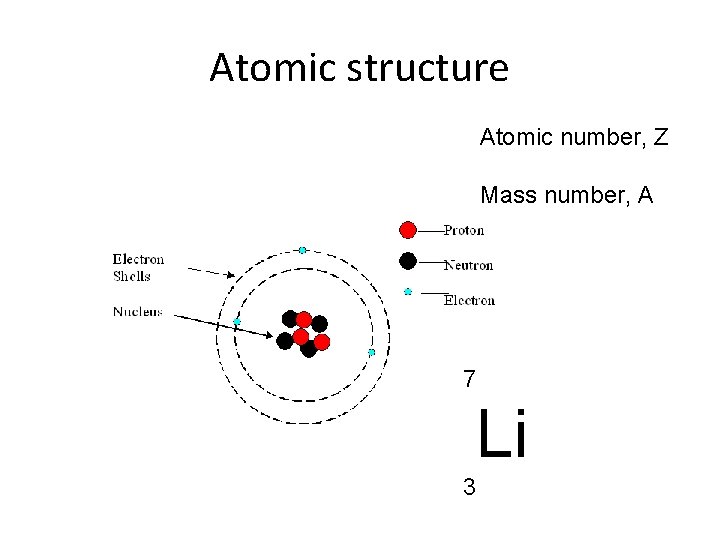
Atomic structure Atomic number, Z Mass number, A 7 Li 3

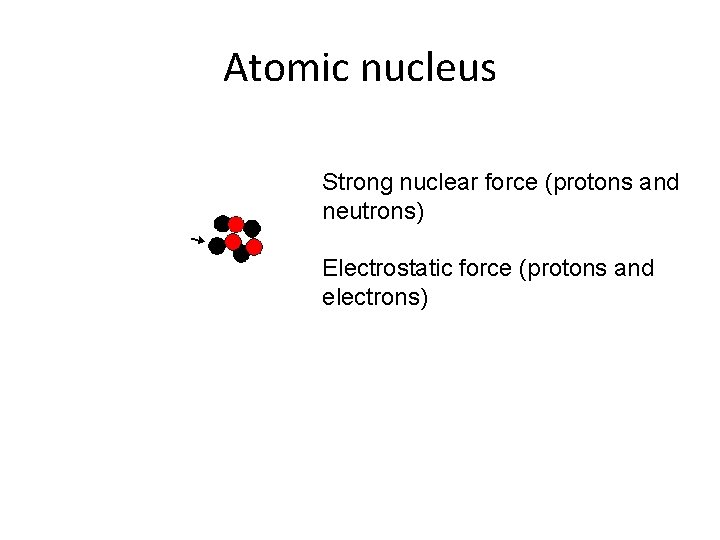
Atomic nucleus Strong nuclear force (protons and neutrons) Electrostatic force (protons and electrons)

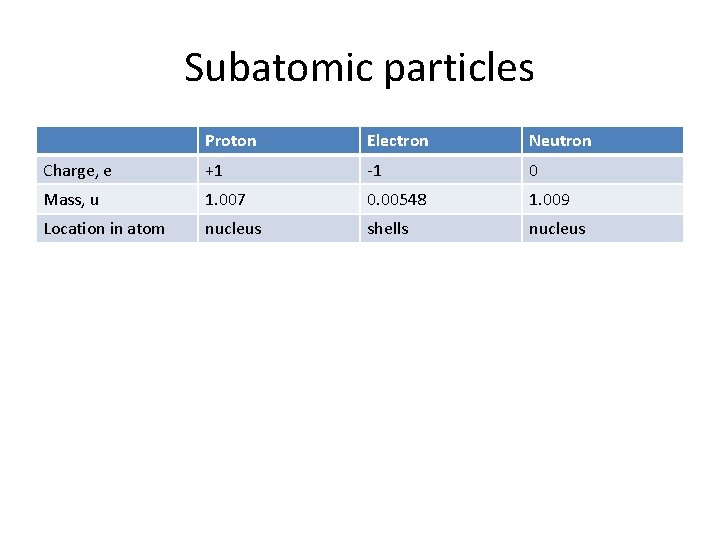
Subatomic particles Proton Electron Neutron Charge, e +1 -1 0 Mass, u 1. 007 0. 00548 1. 009 Location in atom nucleus shells nucleus

ELEMENTARY PARTICLES

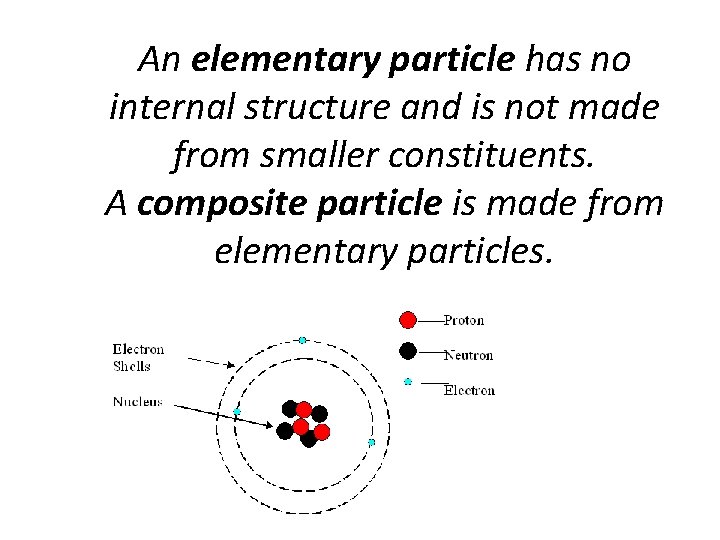
An elementary particle has no internal structure and is not made from smaller constituents. A composite particle is made from elementary particles.

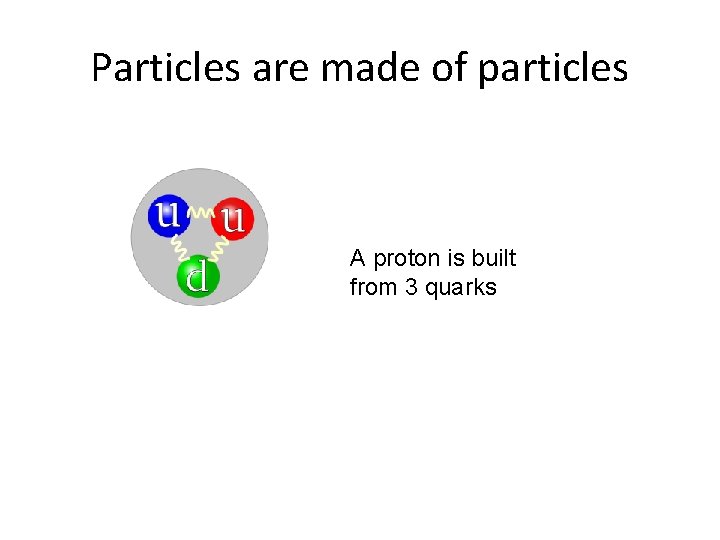
Particles are made of particles A proton is built from 3 quarks

composite: atom proton neutron elementary: electron quark neutrino proton

RADIOACTIVITY

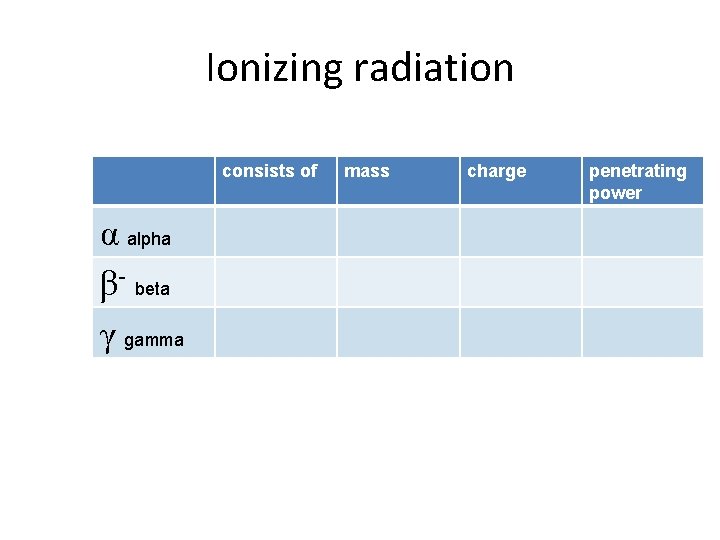
Ionizing radiation consists of α alpha β- beta γ gamma mass charge penetrating power

Background radiation • is the natural radiation from materials in the environment including rocks, the air and living organisms • varies with location

What is the relationship between Z and N? • Research the stable isotopes of as many atoms as possible and plot a graph of N against Z • Plot a trendline and write down the gradient. Explain what the gradient means for this graph. • Explain why this pattern occurs using ideas about the strong nuclear force and the electrostatic force.

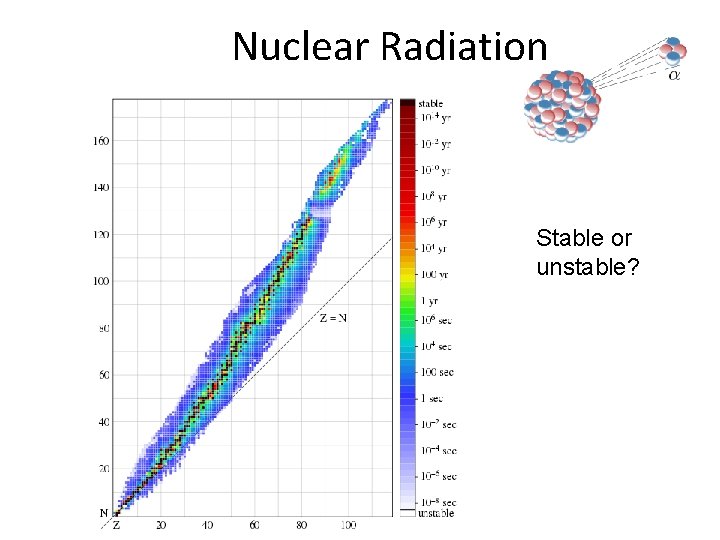
Nuclear Radiation Stable or unstable?

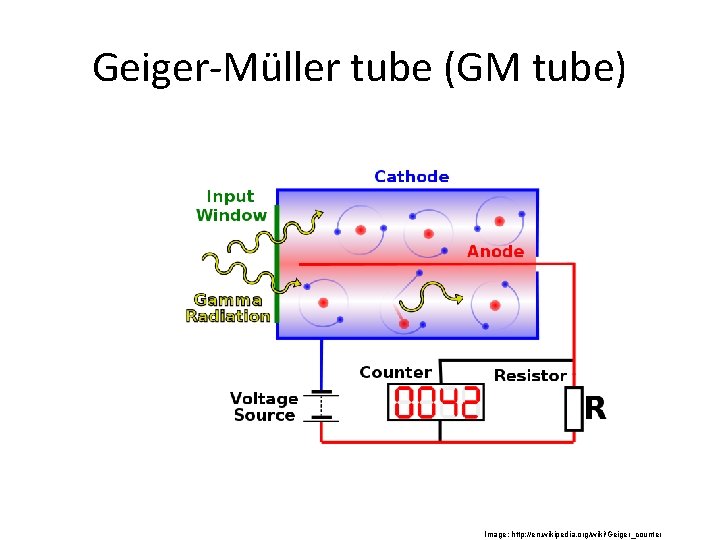
Geiger-Müller tube (GM tube) Image: http: //en. wikipedia. org/wiki/Geiger_counter

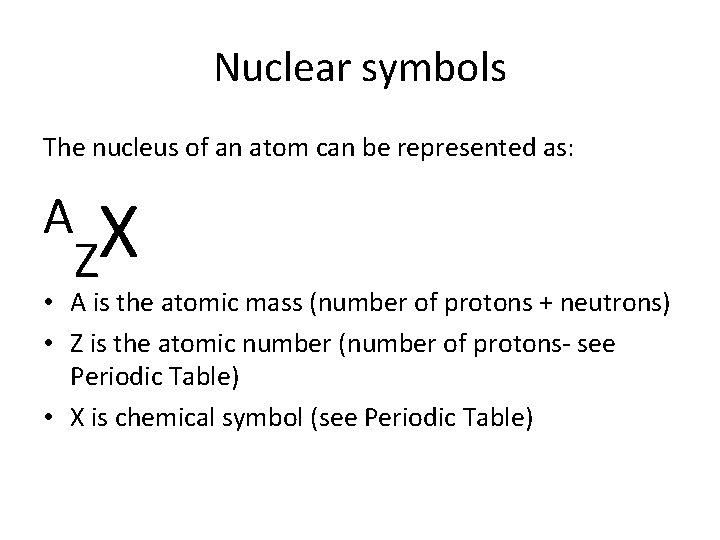
Nuclear symbols The nucleus of an atom can be represented as: A X Z • A is the atomic mass (number of protons + neutrons) • Z is the atomic number (number of protons- see Periodic Table) • X is chemical symbol (see Periodic Table)

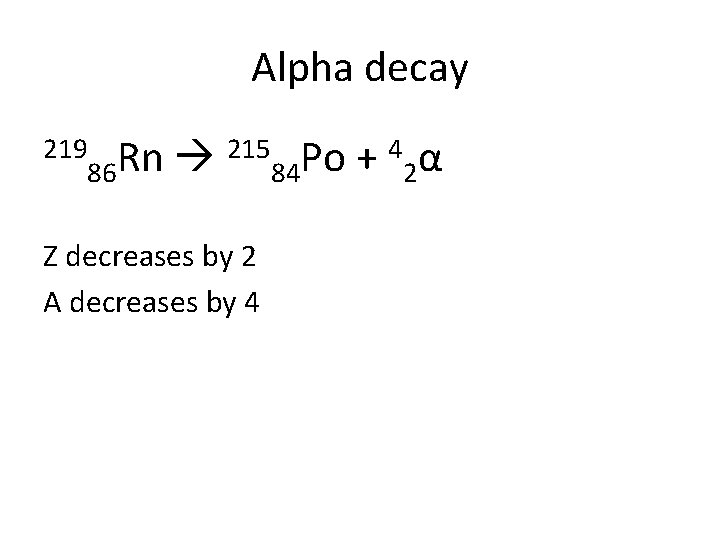
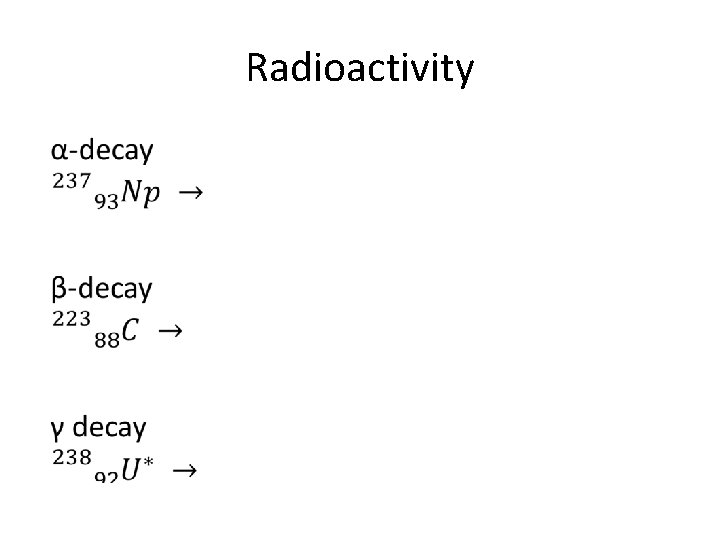
Alpha decay 219 215 Po + 4 α Rn 86 84 2 Z decreases by 2 A decreases by 4

Write nuclear decay equations for the alpha decay of: Polonium-218 Gold-196

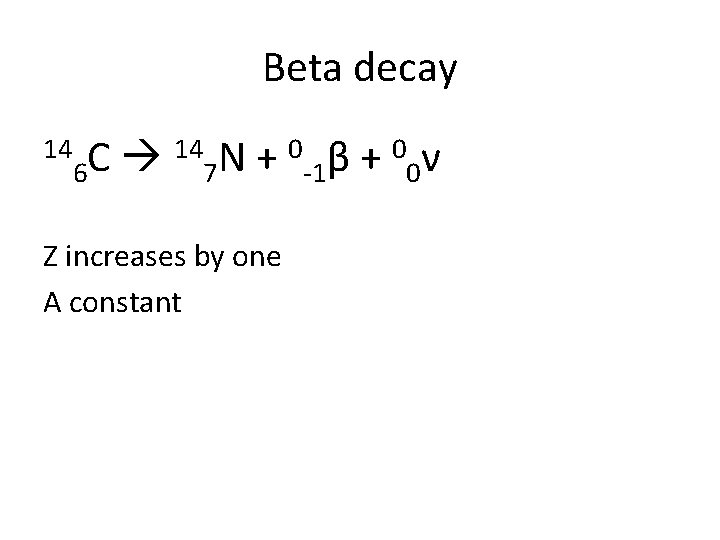
Beta decay 14 14 N + 0 β + 0 ν C 6 7 -1 0 Z increases by one A constant

Write nuclear decay equations for the beta decay of: Phosphorous-32 Iodine-131

Radioactivity •

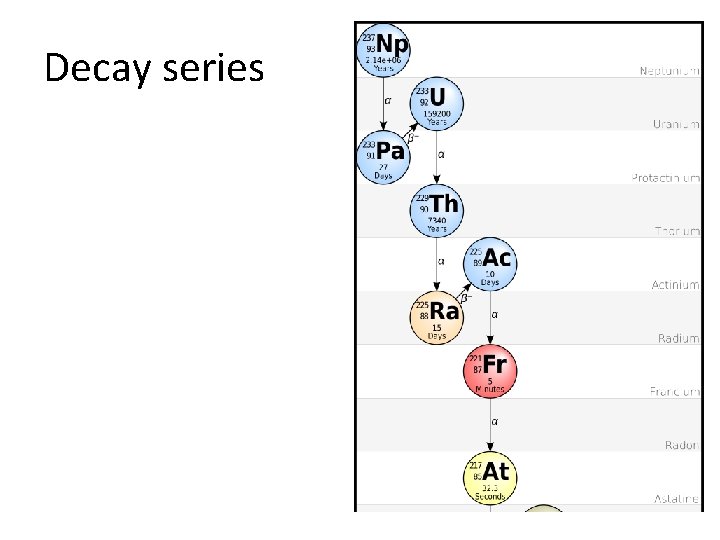
Decay series

HOW LONG DOES IT TAKE AN UNSTABLE ATOM TO DECAY?

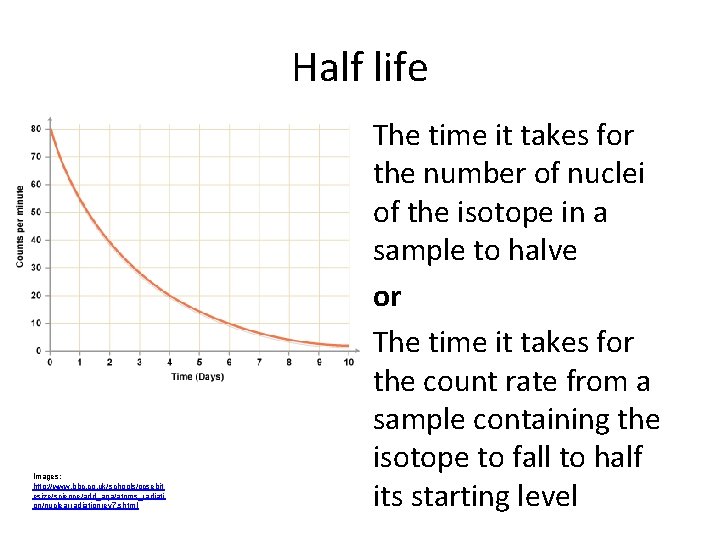
Half life Images: http: //www. bbc. co. uk/schools/gcsebit esize/science/add_aqa/atoms_radiati on/nuclearradiationrev 7. shtml The time it takes for the number of nuclei of the isotope in a sample to halve or The time it takes for the count rate from a sample containing the isotope to fall to half its starting level

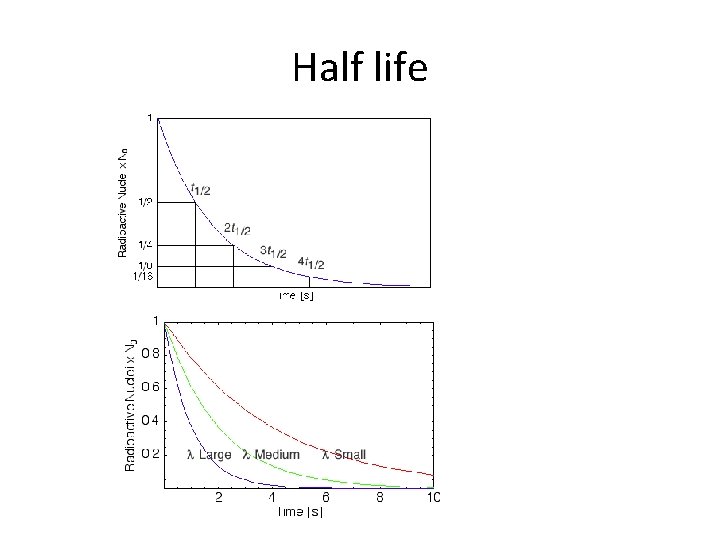
Half life

Activity • The activity of a sample, A, is the number of decays in one second. • The decay constant, λ, is the probability that a single nucleus will decay in one second. • N is the total number of unstable nuclei

Radioactive decay law •

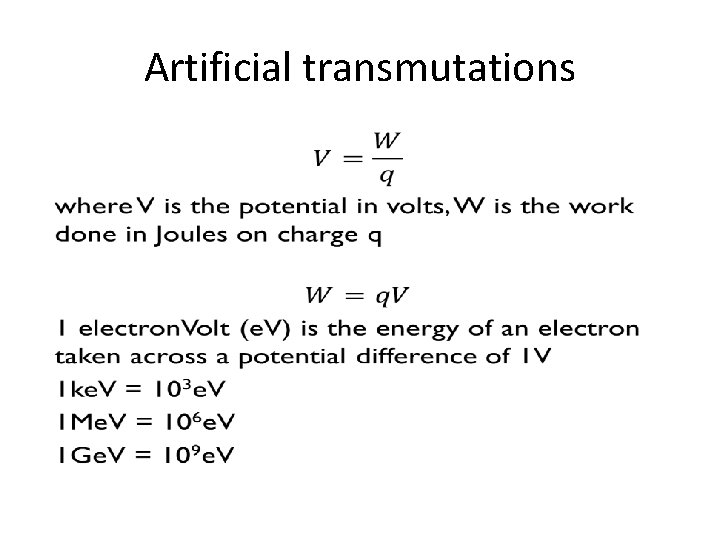
ARTIFICIAL TRANSMUTATION

Artificial transmutations •

NUCLEAR BINDING ENERGY

Bang! Trinity test • plutonium fission • 84 Tera. Joules = 20 kton TNT

Bang! first H bomb test in 1952 • hydrogen fusion • 44 Peta. Joules = 10 Mtons of TNT

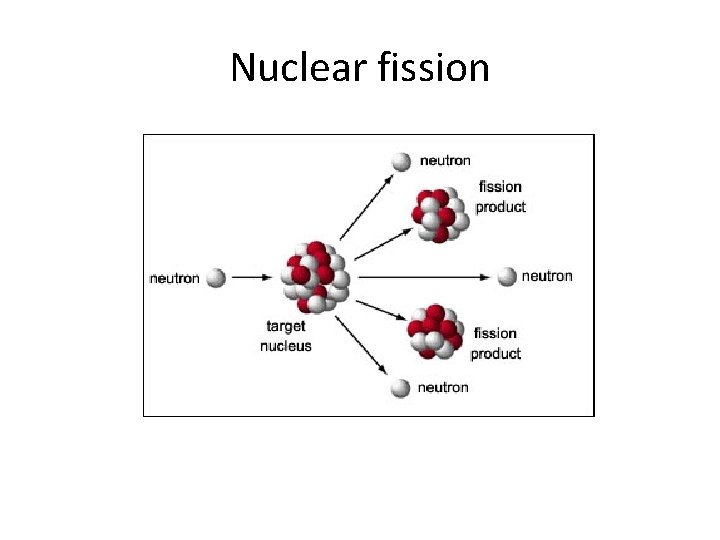
Nuclear fission

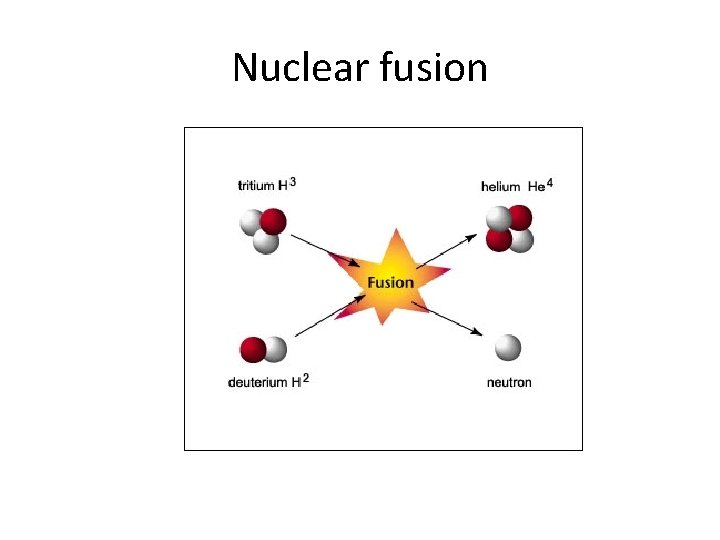
Nuclear fusion

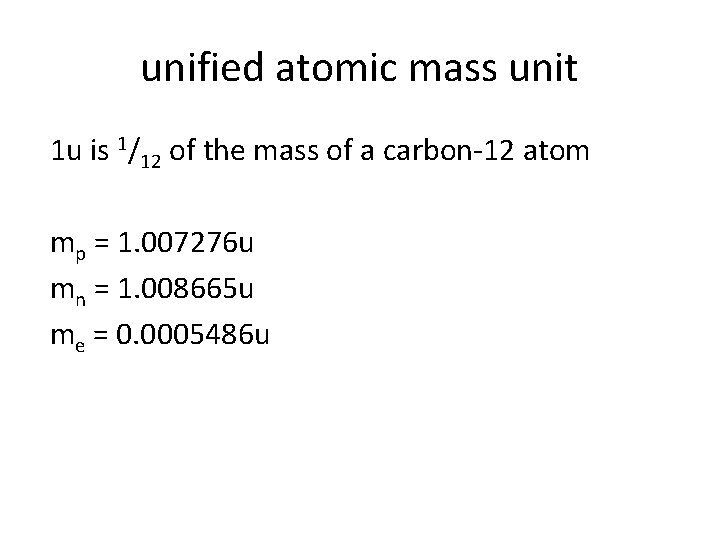
unified atomic mass unit 1 u is 1/12 of the mass of a carbon-12 atom mp = 1. 007276 u mn = 1. 008665 u me = 0. 0005486 u

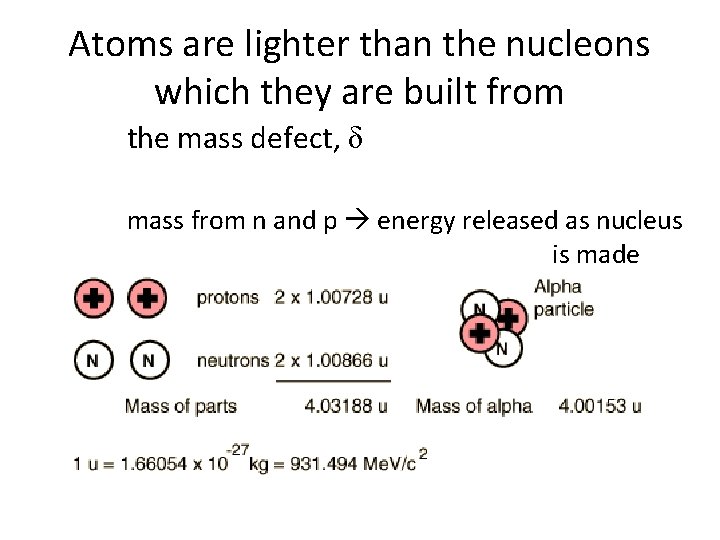
Atoms are lighter than the nucleons which they are built from the mass defect, δ is the difference between the total mass of the individual nucleons and the mass of the atomic nucleus mass defect of Helium m. He = 4. 0026 u

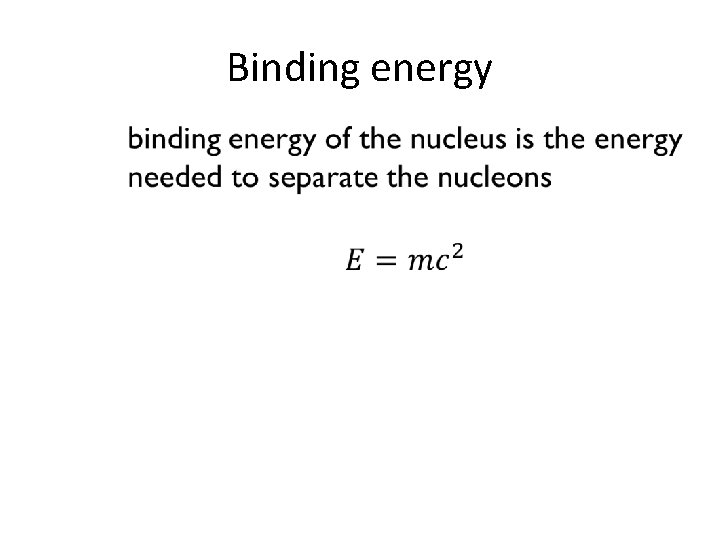
Binding energy •

How much energy is released when a helium nucleus is formed? • • mp = 1. 00728 u mn = 1. 00866 mass of 42 He nucleus = 4. 00153 u 1 u = 1. 66054 x 10 -27 kg = 931. 494 Me. Vc-2

Atoms are lighter than the nucleons which they are built from the mass defect, δ mass from n and p energy released as nucleus is made Tsokos p. 387 qn 1

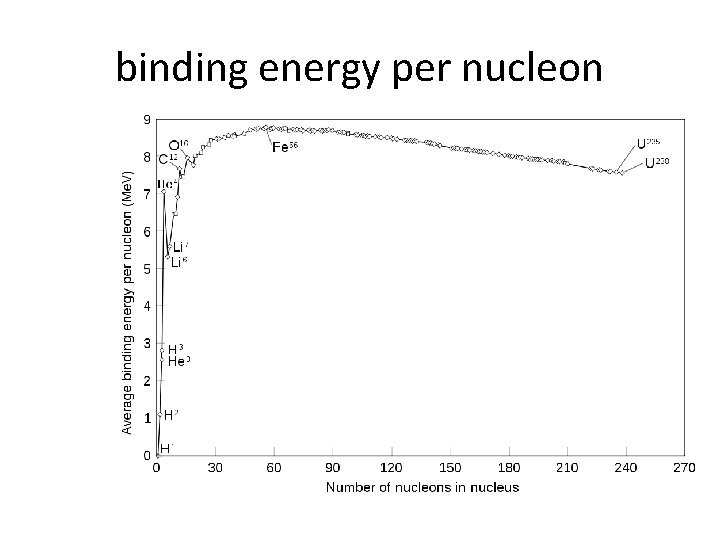
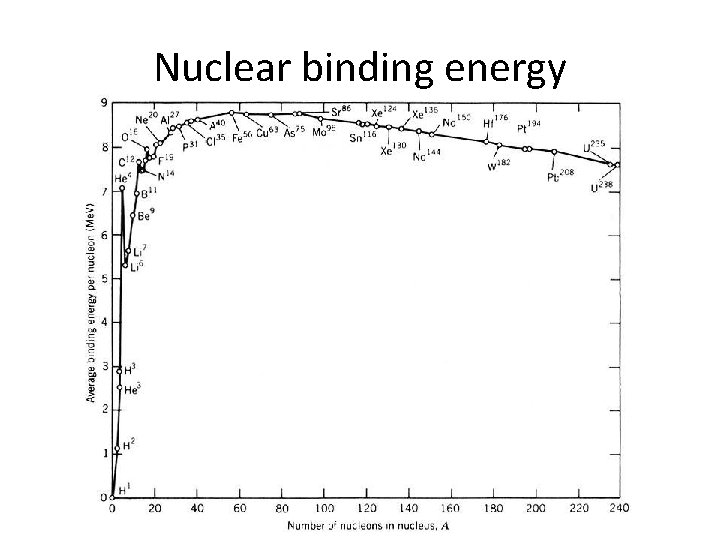
binding energy per nucleon

Fission and fusion How does the curve of binding energy per nucleon explain why fission of heavy elements releases energy, while fusion of light elements releases energy?

NUCLEAR FISSION

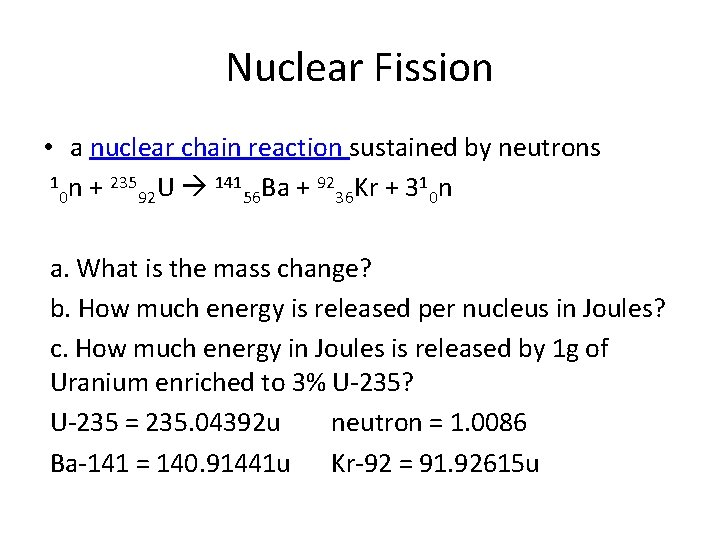
Nuclear Fission • a nuclear chain reaction sustained by neutrons 1 n + 235 U 141 Ba + 92 Kr + 31 n 0 92 56 36 0 a. What is the mass change? b. How much energy is released per nucleus in Joules? c. How much energy in Joules is released by 1 g of Uranium enriched to 3% U-235? U-235 = 235. 04392 u neutron = 1. 0086 Ba-141 = 140. 91441 u Kr-92 = 91. 92615 u

The fission of one atom of U-235 generates 202. 5 Me. V = 3. 244 × 10− 11 J equivalent to 19. 54 TJ/mol = 83. 14 TJ/kg

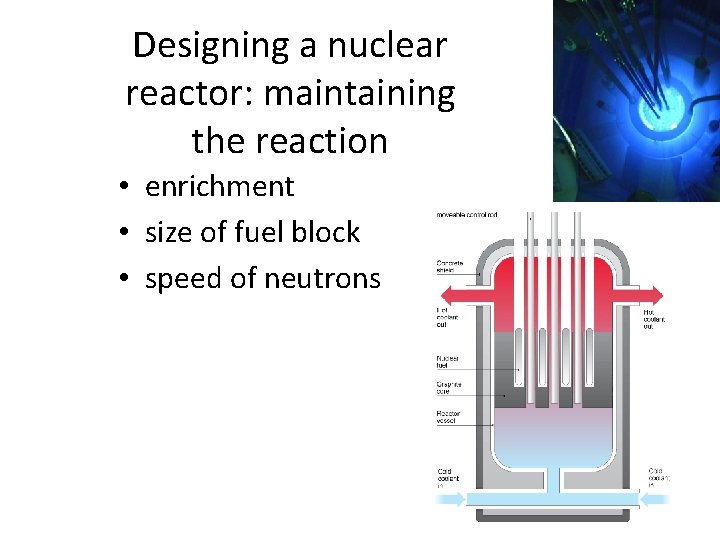
Designing a nuclear reactor: maintaining the reaction • enrichment • size of fuel block • speed of neutrons moderator- slows neutrons control rods- absorb neutrons

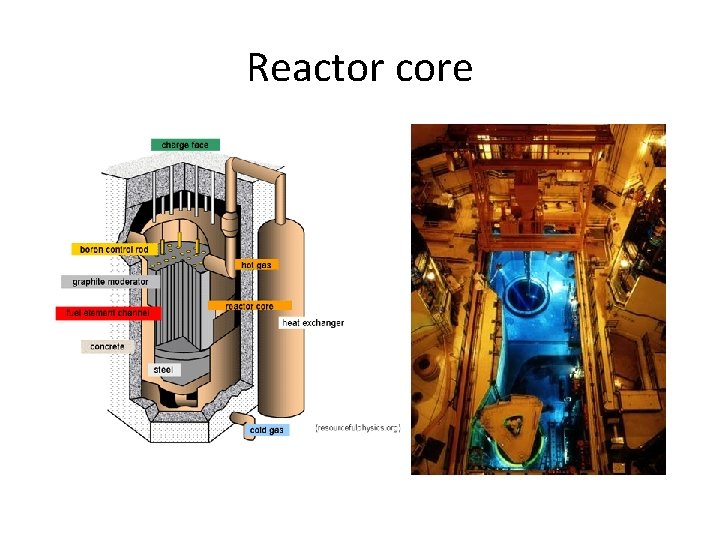
Reactor core

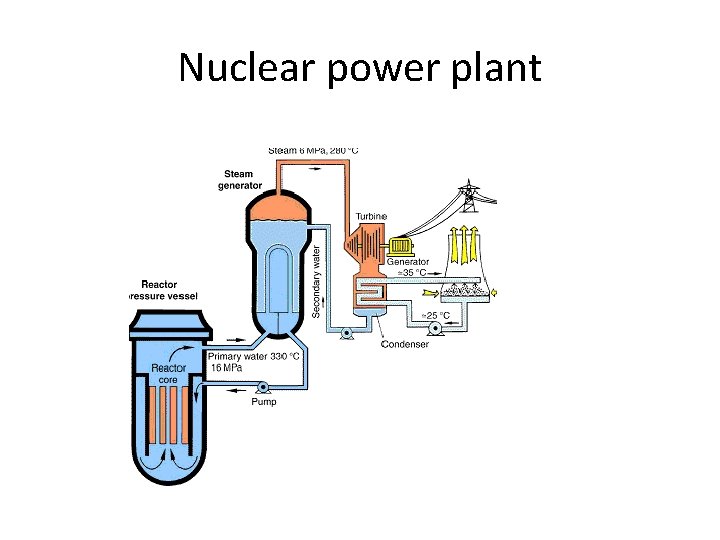
Nuclear power plant Advantages: high energy density huge reserves of U Disadvantages : radioactive waste Accident risk Proliferation Non-renewable

Nuclear waste: mix of isotopes with short and long half lives spent fuel in a storage pond underground storage

Production of Plutonium-239 1 n 239 U U + 92 0 92 239 U 239 Np + 0 β + 0 ν 92 93 -1 0 239 Np 239 Pu + 0 β + 0 ν 93 94 -1 0 238 plutonium is toxic, but can be reprocessed and used in a fast breeder reactor (or for a fission bomb)

NUCLEAR FUSION

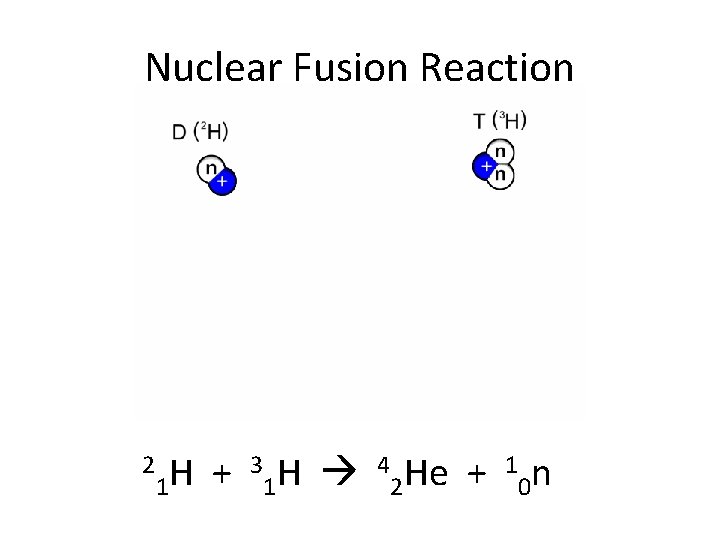
Nuclear Fusion Reaction 2 3 H 4 He + 1 n H + 1 1 2 0

How much energy is released in nuclear fusion? Deuterium Hydrogen Helium-4 neutron 2. 01410 u 1. 00783 u 4. 00260 u 1. 0086 u

Nuclear binding energy

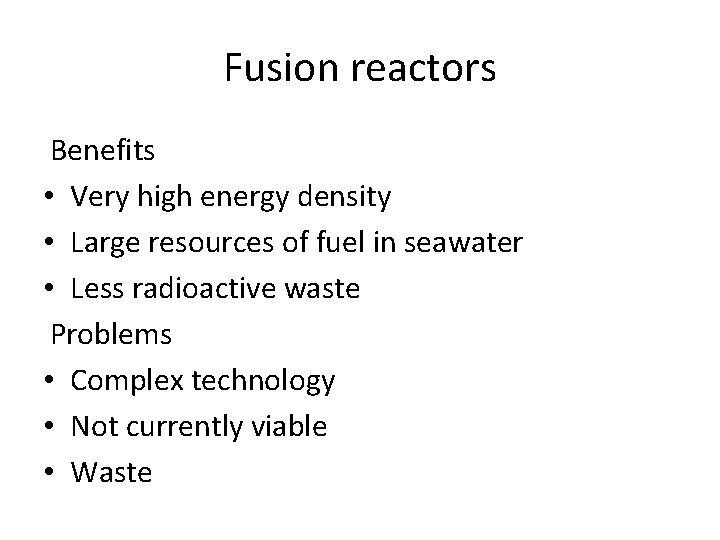
Fusion reactors Benefits • Very high energy density • Large resources of fuel in seawater • Less radioactive waste Problems • Complex technology • Not currently viable • Waste

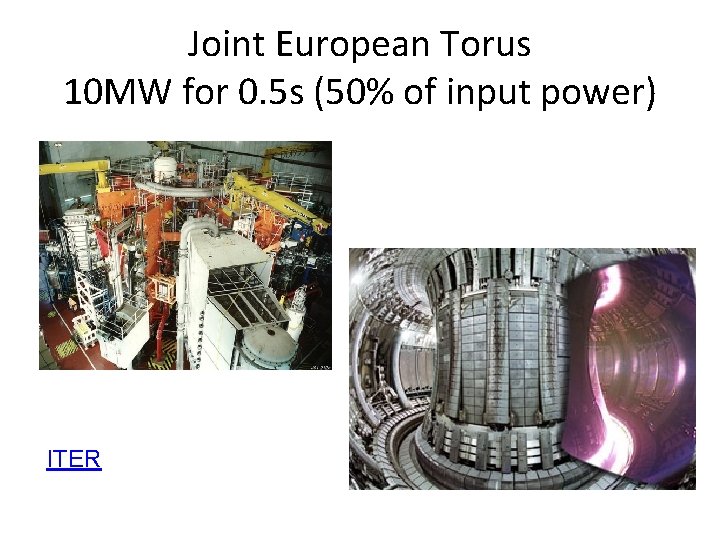
Joint European Torus 10 MW for 0. 5 s (50% of input power) 3 m radius 3. 45 T magnetic ITERfield current 3 MA

Teaching notes