PD 233 Design of Biomedical Devices and Systems
PD 233: Design of Biomedical Devices and Systems (Lecture 4 ) Dr. Manish Arora CPDM, IISc Course Website: http: //cpdm. iisc. ac. in/utsaah/courses/

Quick Review • Definition & classification of Medical Devices • Bioethics in Medical Device Innovation
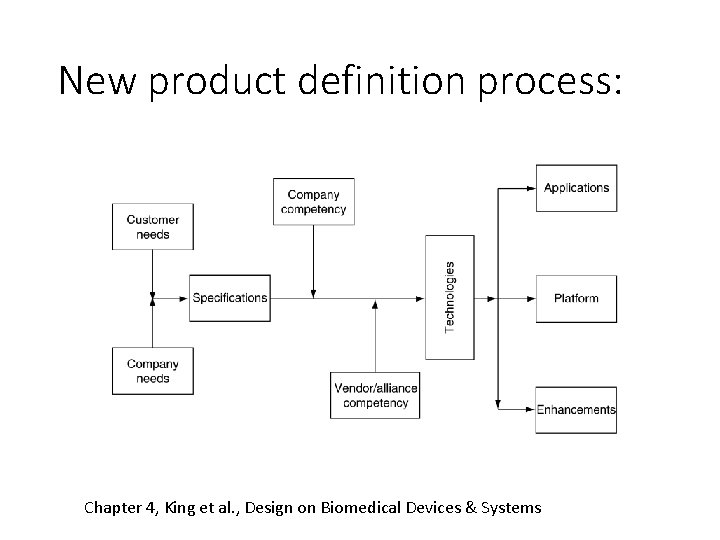
New product definition process: Chapter 4, King et al. , Design on Biomedical Devices & Systems

Capturing Customer Needs: • Chance of success are much higher when there is ‘Market Pull’ vs ‘Technology Push’ • Design is about solving an “unmet need” • How to find unmet need?

How to find unmet clinical need? • Look in scientific journal papers. . • Look in newspapers. . • Ask the doctor. . • Ask the patient. . • ‘Immersion’ in field (PHC, Polyclinics, Hospitals) • A lot of problems can be identified by careful observation in the real-life practice. • Filter based on your competencies and interest
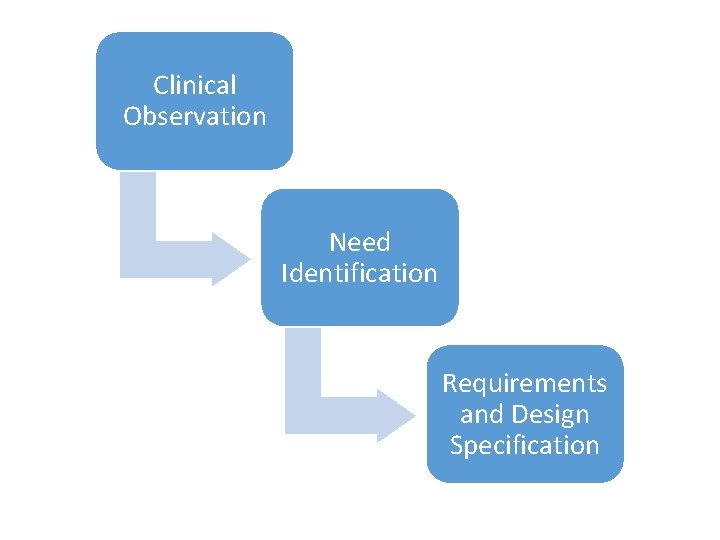
Clinical Observation Need Identification Requirements and Design Specification

Requirement and Design Specification Both requirements and design specification needs for be documented for regulatory approval • Requirements deals with what needs to be done “hard copy of strip chart is made available to clinician’ • Design specification deals with how it is done “hard copy of the strip chart is made available to clinician by pressing menu key”
Verification and Validation Another big piece of regulatory approvals deals with documentation for Verification & Validations. Verification is process of ensuring all requirements are met at that stage of design. -Can be done at sub-system as well as system level. Validation is process of evaluating product to ensure compliance with specified and implied requirements -Validation is preformed at end of design cycle on actual device as manufactured according to manufacturing specifications and standards. Both need to be documented fully
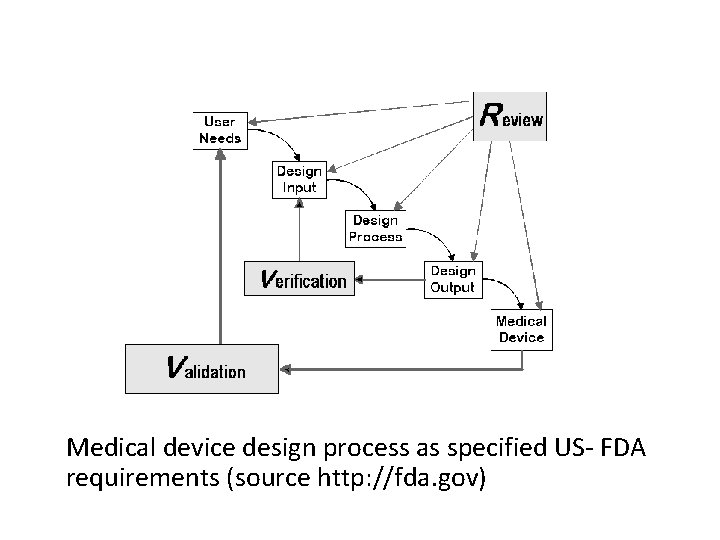
Medical device design process as specified US- FDA requirements (source http: //fda. gov)
- Slides: 9