PCR mediated mutagenesis 2013 2 2 6 Introduction




- Slides: 18

PCR mediated mutagenesis 2013년도 2학기 생화학 실험 (2) 6주차 조교 : 전지선

Introduction. Site-specific mutagenesis • The cause of mutagenesis. • The types of mutagenesis. 1. UV. 1. Single base mutation. 2. Chemical-Carcinogen. 2. Multiple mutation. 3. Error prone of PCR. 3. Insertion. 4. Site-directed mutagenesis. 4. Deletion.

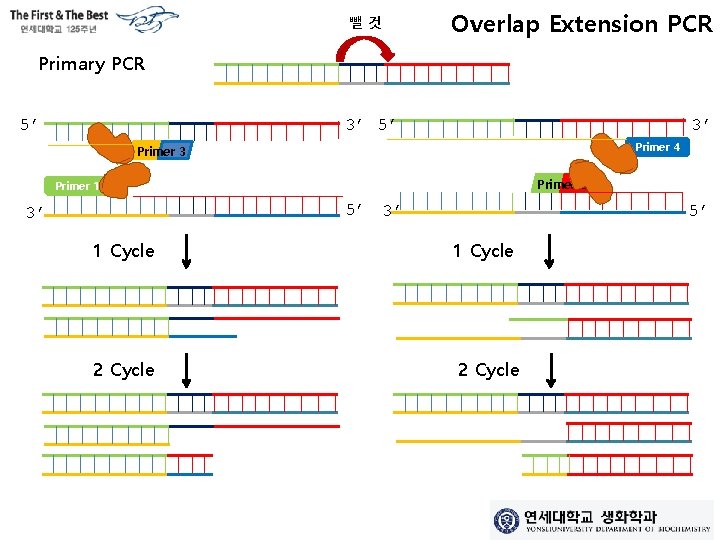
뺄것 Overlap Extension PCR Primary PCR 5’ 3’ Primer 4 Primer 3 Primer 2 Primer 1 5’ 3’ 3’ 5’ 1 Cycle 2 Cycle

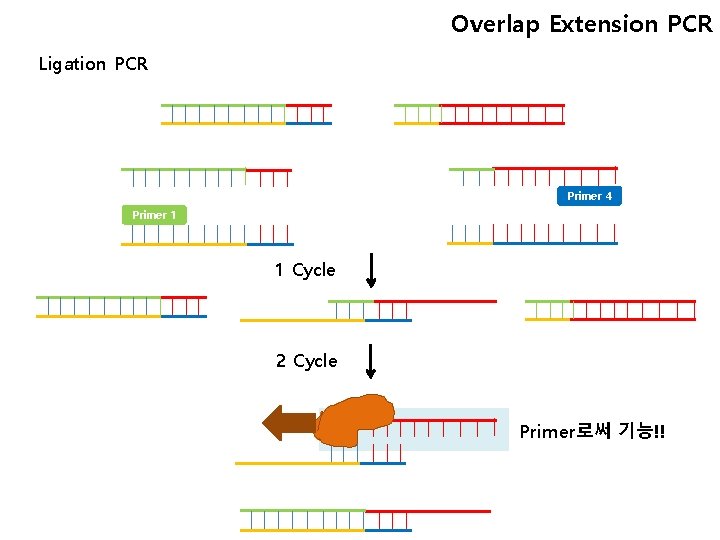
Overlap Extension PCR Ligation PCR Primer 4 Primer 1 1 Cycle 2 Cycle Primer로써 기능!!

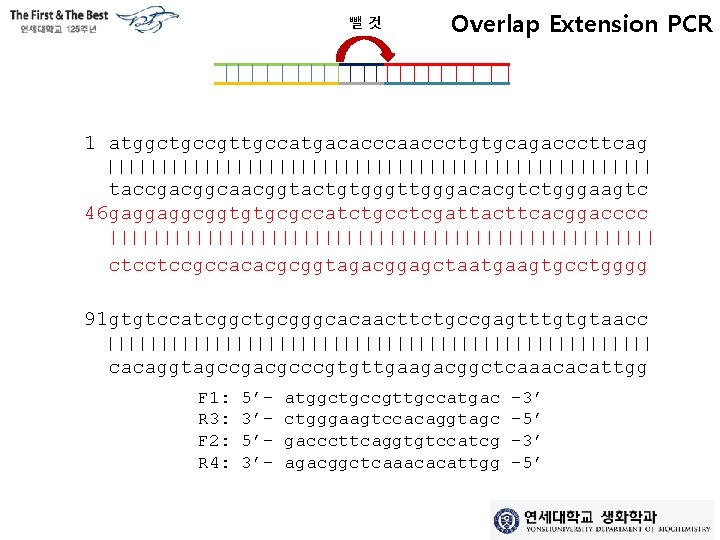
뺄것 Overlap Extension PCR 1 atggctgccgttgccatgacacccaaccctgtgcagacccttcag llllllllllllllllllllllllll taccgacggcaacggtactgtgggttgggacacgtctgggaagtc 46 gaggaggcggtgtgcgccatctgcctcgattacttcacggacccc llllllllllllllllllllllllll ctcctccgccacacgcggtagacggagctaatgaagtgcctgggg 91 gtgtccatcggctgcgggcacaacttctgccgagtttgtgtaacc llllllllllllllllllllllllll cacaggtagccgacgcccgtgttgaagacggctcaaacacattgg F 1: R 3: F 2: R 4: 5’ 3’- atggctgccgttgccatgac ctgggaagtccacaggtagc gacccttcaggtgtccatcg agacggctcaaacacattgg -3’ -5’

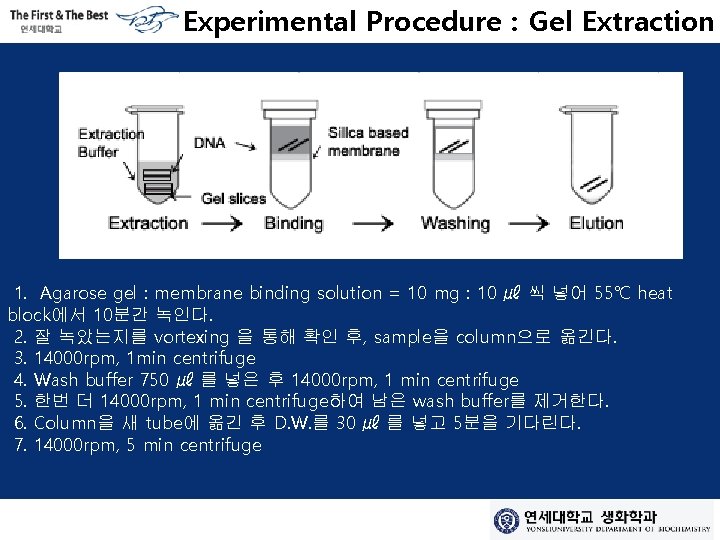
Experimental Procedure : Gel Extraction 1. Agarose gel : membrane binding solution = 10 mg : 10 ㎕ 씩 넣어 55℃ heat block에서 10분간 녹인다. 2. 잘 녹았는지를 vortexing 을 통해 확인 후, sample을 column으로 옮긴다. 3. 14000 rpm, 1 min centrifuge 4. Wash buffer 750 ㎕ 를 넣은 후 14000 rpm, 1 min centrifuge 5. 한번 더 14000 rpm, 1 min centrifuge하여 남은 wash buffer를 제거한다. 6. Column을 새 tube에 옮긴 후 D. W. 를 30 ㎕ 를 넣고 5분을 기다린다. 7. 14000 rpm, 5 min centrifuge

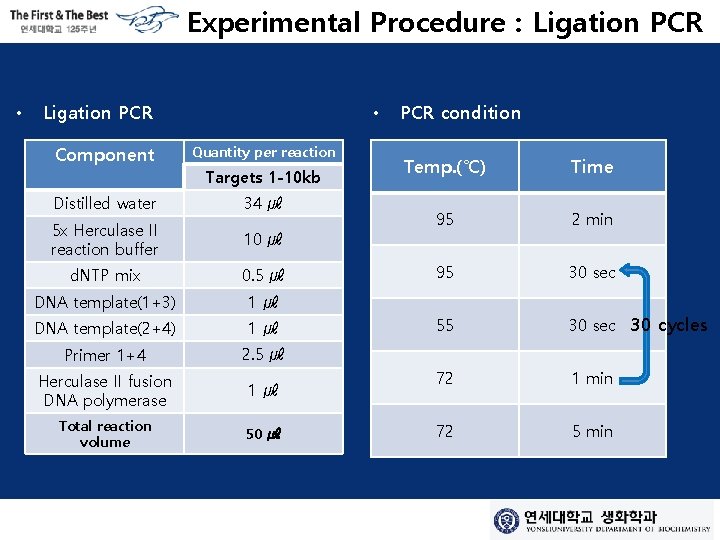
Experimental Procedure : Ligation PCR • Ligation PCR Component • Quantity per reaction Targets 1 -10 kb Distilled water 34 ㎕ 5 x Herculase II reaction buffer 10 ㎕ d. NTP mix 0. 5 ㎕ DNA template(1+3) 1㎕ DNA template(2+4) 1㎕ Primer 1+4 2. 5 ㎕ Herculase II fusion DNA polymerase 1㎕ Total reaction volume 50 ㎕ PCR condition Temp. (℃) Time 95 2 min 95 30 sec 55 30 sec 30 cycles 72 1 min 72 5 min

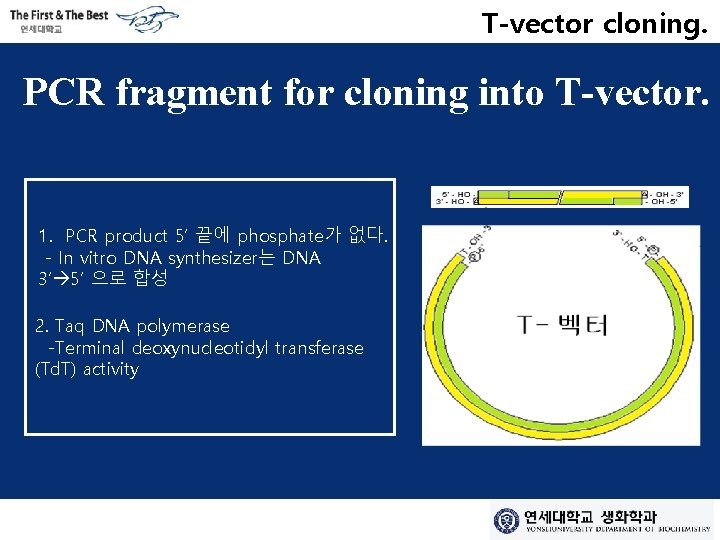
T-vector cloning. PCR fragment for cloning into T-vector. 1. PCR product 5’ 끝에 phosphate가 없다. - In vitro DNA synthesizer는 DNA 3’ 5’ 으로 합성 2. Taq DNA polymerase -Terminal deoxynucleotidyl transferase (Td. T) activity

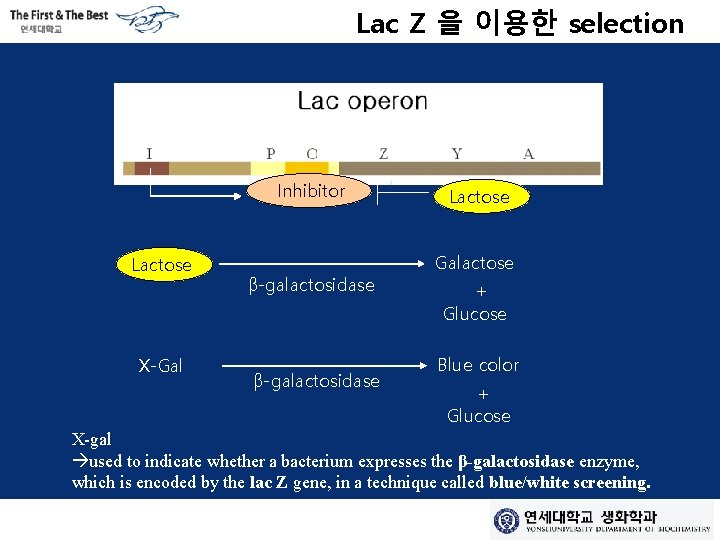
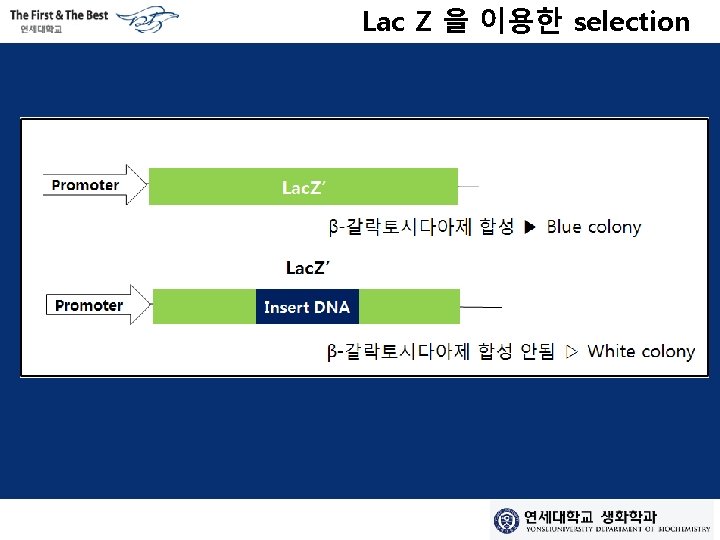
Lac Z 을 이용한 selection Inhibitor Lactose X-Gal β-galactosidase Lactose Galactose + Glucose Blue color + Glucose X-gal used to indicate whether a bacterium expresses the β-galactosidase enzyme, which is encoded by the lac Z gene, in a technique called blue/white screening.

Lac Z 을 이용한 selection

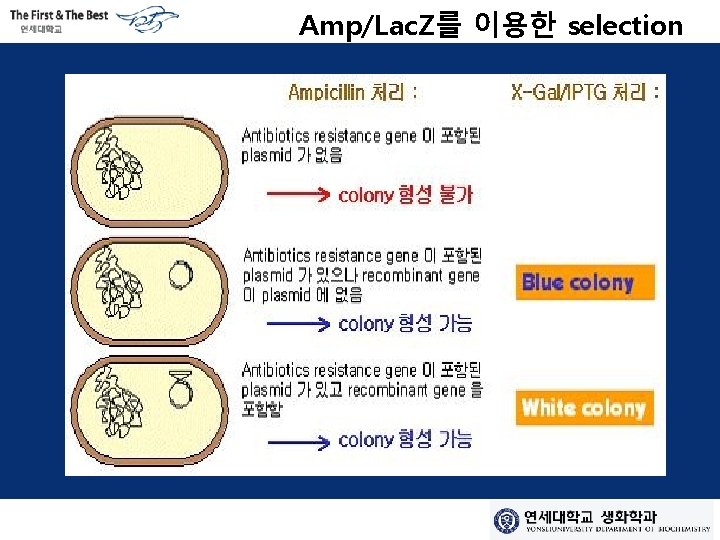
Amp/Lac. Z를 이용한 selection

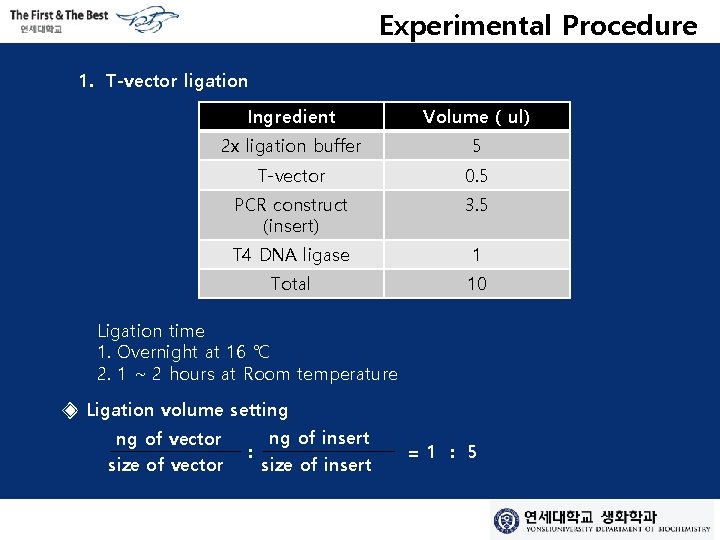
Experimental Procedure 1. T-vector ligation Ingredient Volume ( ul) 2 x ligation buffer 5 T-vector 0. 5 PCR construct (insert) 3. 5 T 4 DNA ligase 1 Total 10 Ligation time 1. Overnight at 16 ℃ 2. 1 ~ 2 hours at Room temperature ◈ Ligation volume setting ng of vector size of vector : ng of insert size of insert =1 : 5

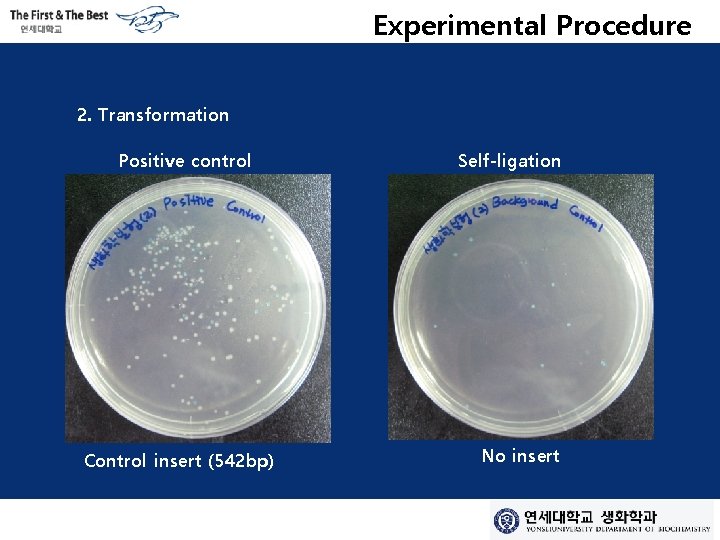
Experimental Procedure 2. Transformation Positive control Control insert (542 bp) Self-ligation No insert

Experimental Procedure 3. Bacteria culture 16 hr 4. Plasmid DNA Extraction 5. Cloning confirmation using two-cut digestion

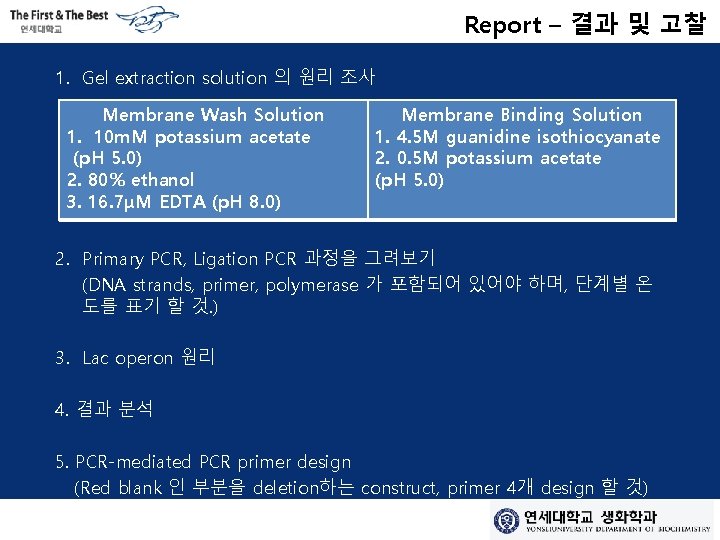
Report – 결과 및 고찰 1. Gel extraction solution 의 원리 조사 Membrane Wash Solution 1. 10 m. M potassium acetate (p. H 5. 0) 2. 80% ethanol 3. 16. 7μM EDTA (p. H 8. 0) Membrane Binding Solution 1. 4. 5 M guanidine isothiocyanate 2. 0. 5 M potassium acetate (p. H 5. 0) 2. Primary PCR, Ligation PCR 과정을 그려보기 (DNA strands, primer, polymerase 가 포함되어 있어야 하며, 단계별 온 도를 표기 할 것. ) 3. Lac operon 원리 4. 결과 분석 5. PCR-mediated PCR primer design (Red blank 인 부분을 deletion하는 construct, primer 4개 design 할 것)

Report - 결과 및 고찰 1 atggctgccgttgccatgacacccaaccctgtgcagacccttcag 46 gaggaggcggtgtgcgccatctgcctcgattacttcacggacccc 91 gtgtccatcggctgcgggcacaacttctgccgagtttgtgtaacc 136 cagttgtggggaggatgaggaggacagagatgagttagat 181 cgggaggaggacggagaggaggaggaagtggaggct 226 gtgggggctggcgcggggtgggacacccccatgcgggatgaagac 271 tacgagggtgacatggaggaggaggtcgaggaggaagaagagggt 316 gtgttctggaccagtggcatgagcaggtccagctgggacaacatg 361 gactatgtgtgggaggacgaggaggaagacctggactac 406 tacttgggggacatggaggacctgaggggggaggatgag 451 gaggacgaggaggaagtgctggaggaggttgaggaagaggatcta 496 gaccccgtcaccccactgcccccgcctccagcccctcggaggtgc 541 ttcacatgccctcagtgccgaaagagctttcctcggcggagcttc 586 cgccccaacctgcagctggccaatatggtccaggtgattcggcag 631 atgcacccaacccctggtcgagggagccgcgtgaccgatcagggc 676 atctgtcccaaacaccaagaagccctgaagctcttctgcgaggta 721 gacgaagaggccatctgtgtgccgagaatccaggagccac 766 aaacagcgtggtgccattggaggaggtggtgcaggagtac 811 aaggccaaactgcaggggcacgtggaaccactgaggaagcacctg 856 gaggcagtgcagaagatgaaagccaaggaggagaggcgagtgaca 901 gaactgaagagccagatgaagtcagagctggcagcggtggcctcg 946 gagtttgggcgactgacacggtttctggctgaagagcaggg 991 ctggaacggcgtctcagagagatgcatgaagcccagctggggcgt 1036 gcgggagccgcggctagtcgccttgcagaacaggccgcccagctc 1081 agccgcctgctggcagaggcccaggagccagcaggggggt 1126 ctccggctgctccaggacatcaaggagactttcaataggtgtgaa

