PCR mediated mutagenesis 2013 2 2 5 Procedure













- Slides: 22

PCR mediated mutagenesis 2013년도 2학기 생화학 실험 (2) 5주차 조교 : 안성원

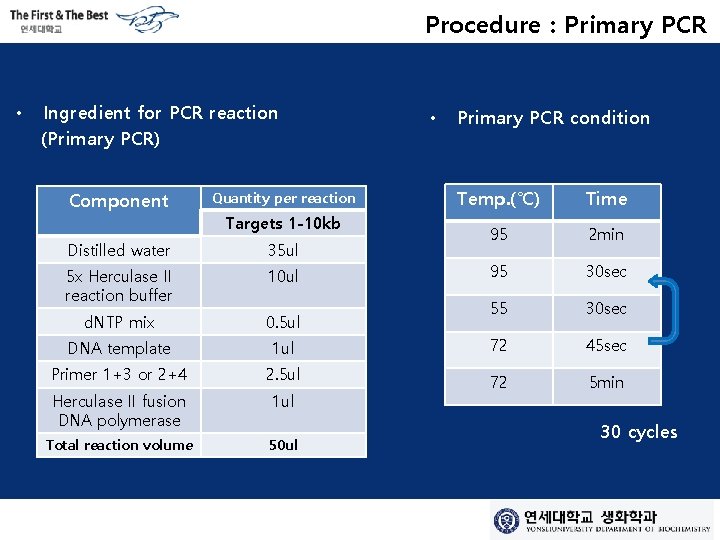
Procedure : Primary PCR • Ingredient for PCR reaction (Primary PCR) Component Quantity per reaction Targets 1 -10 kb • Primary PCR condition Temp. (℃) Time 95 2 min 95 30 sec 55 30 sec Distilled water 35 ul 5 x Herculase II reaction buffer 10 ul d. NTP mix 0. 5 ul DNA template 1 ul 72 45 sec Primer 1+3 or 2+4 2. 5 ul Herculase II fusion DNA polymerase 1 ul 72 5 min Total reaction volume 50 ul 30 cycles


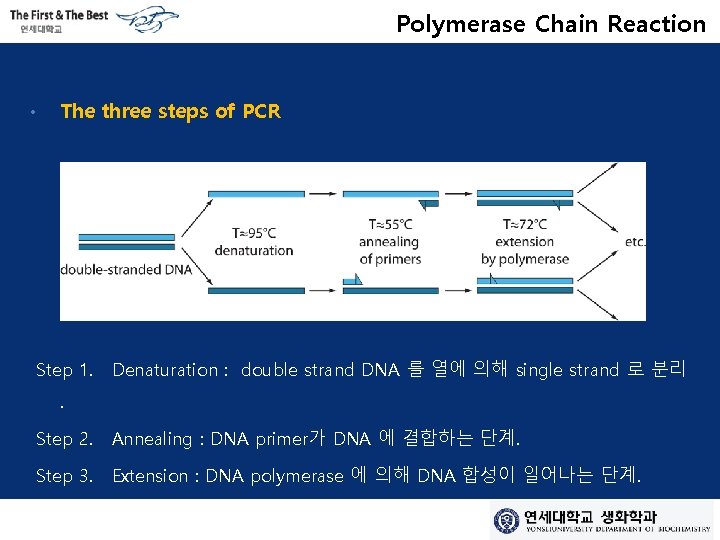
Polymerase Chain Reaction • The three steps of PCR Step 1. Denaturation : double strand DNA 를 열에 의해 single strand 로 분리 . Step 2. Annealing : DNA primer가 DNA 에 결합하는 단계. Step 3. Extension : DNA polymerase 에 의해 DNA 합성이 일어나는 단계.

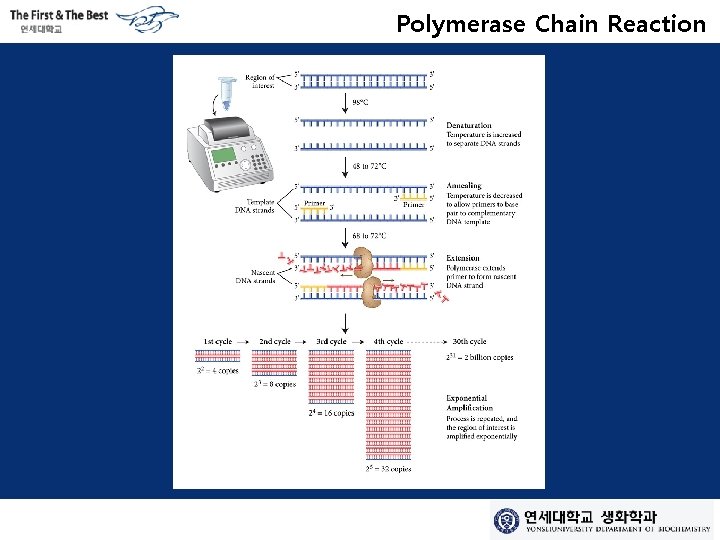
Polymerase Chain Reaction

Polymerase Chain Reaction

Polymerase Chain Reaction

Applications of PCR • Sub-cloning DNA targets using PCR • PCR-meditated in vitro mutagenesis – Site-directed mutagenesis (위치 선택적 돌연변이) – Overlap Extension PCR • Amplification of differentially expressed gene sequences – RT-PCR • Identifying genetic mutation – Reverse Genetics Approach • etc……


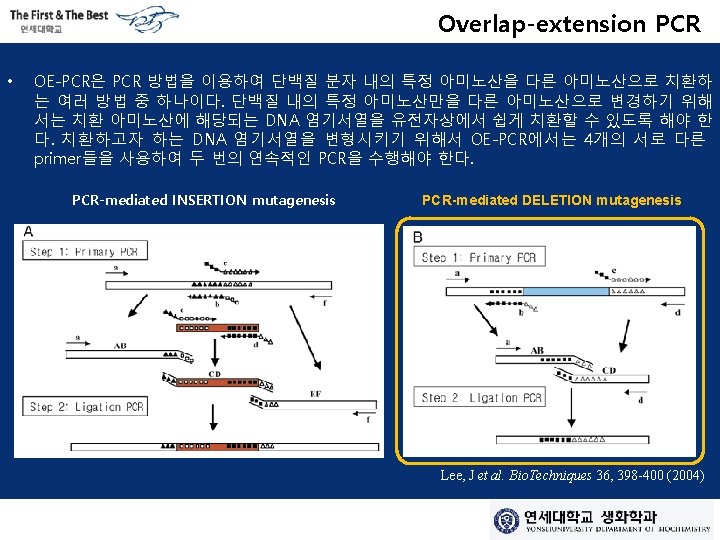
Primer • Primary PCR using chimeric primers – Primer A + Primer B (chimeric primer with primer C sequence) – Primer D + Primer C (chimeric primer with primer B sequence) – Primer B COMPLEMENTARY SEQUENCE Primer C • PCR construct purification through gel elution – Intermediate PCR construct (A-B & C-D) • Ligation PCR using outer primers (A and D) – Using Intermediate PCR construct (A-B + C-D) as a DNA template – Final product is mutagenized PCR construct which has deleted region



Primer Homo sapiens tripartite motif containing 41 (TRIM 41), transcript variant 2, m. RNA 586 631 676 721 766 811 • • cgccccaacctgcagctggccaatatggtccaggtgattcggcag atgcacccaacccctggtcgagggagccgcgtgaccgatcagggc atctgtcccaaacaccaagaagccctgaagctcttctgcgaggta gacgaagaggccatctgtgtgccgagaatccaggagccac aaacagcgtggtgccattggaggaggtggtgcaggagtac aaggccaaactgcaggggcacgtggaaccactgaggaagcacctg RINCK 1 -F : 5’ atggctgccgttgccatgac 3’ RINCK 1 -BB-F : 5’ GGTCGAGGGAGCCGCGTG-GAGGAGGTGGTGCAGGAG 3’ RINCK 1 -BB-R : 5’ CTCCTGCACCACCTCCTC CACGCGGCTCCCTCGACC 3’ RINCK 1 -R : 5’tcaagtgaagctaaattctg 3’

Primer

Procedure : Primary PCR • Ingredient for PCR reaction (Primary PCR) Component Quantity per reaction Targets 1 -10 kb • Primary PCR condition Temp. (℃) Time 95 2 min 95 30 sec 55 30 sec Distilled water 35 ul 5 x Herculase II reaction buffer 10 ul d. NTP mix 0. 5 ul DNA template 1 ul 72 45 sec Primer 1+3 or 2+4 2. 5 ul Herculase II fusion DNA polymerase 1 ul 72 5 min Total reaction volume 50 ul 30 cycles

Agarose gel electrophoresis of DNA

Gel extraction Gel Slice and PCR Product Preparation Dissolving the Gel Slice 1. Following electrophoresis, excise DNA band from gel and place gel slice in a 1. 5 ml microcentrifuge tube. 2. Add 10μl Membrane Binding Solution per 10 mg of gel slice. Vortex and incubate at 50– 65°C until gel slice is completely dissolved(10 min). Binding of DNA 1. Attach Vacuum Adapter to manifold port and insert SV Minicolumn into Adapter. 2. Transfer dissolved gel mixture or prepared PCR product to the Minicolumn. Incubate at room temperature for 1 minute. 3. Apply vacuum to pull liquid through Minicolumn. Release vacuum when all liquid has passed through Minicolumn. (12, 000 rpm, 1 min) Washing 4. Add 700μl Membrane Wash Solution (ethanol added). Apply a vacuum to pull solution through Minicolumn. . (12, 000 rpm, 1 min → 12, 000 rpm, 1 min 30 sec~2 min Et. OH 날라가도록) 5. Transfer Minicolumn to a Collection Tube. Centrifuge at 16, 000 × g for 5 minutes. 6. Empty the Collection Tube and recentrifuge the column assembly for 1 minute with the microcentrifuge lid open (or off) to allow evaporation of any residual ethanol. Elution 7. Carefully transfer Minicolumn to a clean 1. 5 ml microcentrifuge tube. 8. Add 50μl of Nuclease-Free Water to the Minicolumn. Incubate at room temperature for 5 minute. Centrifuge 12, 000 rpm, 3 min 9. Discard Minicolumn and store DNA at 4°C or – 20°C.

Gel extraction

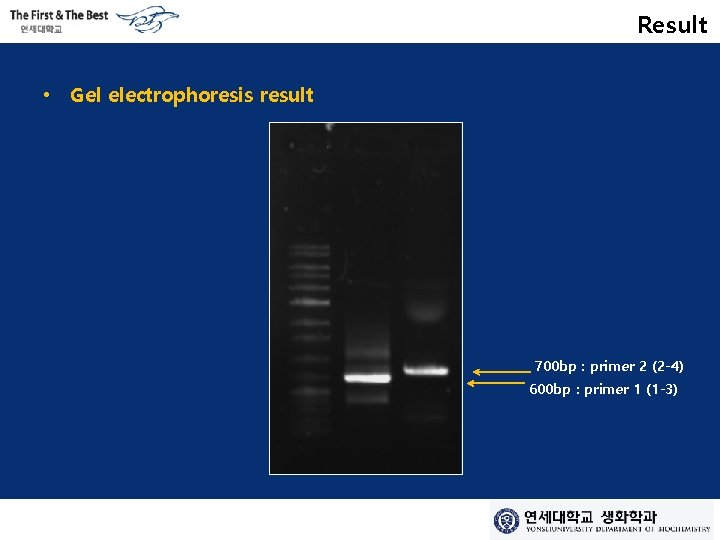
Result • Gel electrophoresis result 700 bp : primer 2 (2 -4) 600 bp : primer 1 (1 -3)



Report – 결과 및 고찰 5. 보편적인 PCR Primer design → 아래의 sequence를 이용해 forward primer와 reverse primer를 설계하세요. primer design할 때 주의사항을 염두하여 20~25 bp사이로 설계한 후 짜여진 두 primer의 Tm값도 구하세요. Gene Description Homo sapiens corepressor interacting with RBPJ, 1 (CIR 1), m. RNA. 299 aaggagccccac gagaaaaata tgccaaagat gacatgaaca tcagagatca gccctttggt 361 attcaggttc gaaatgtgag gtgcattaaa tgtcacaaat ggggtcatgt caacacagat 421 cgagaatgtc ctttgg tctttctgga atcaatgcaa gttcggt