Pauli Exclusion Principle Paulis Exclusion Principle Every electron




- Slides: 15

Pauli Exclusion Principle Pauli’s Exclusion Principle Every electron does its “own thing” 1

Pauli Exclusion Principle Summary of Bohr’s Model (1913) Electrons are in different orbits at fixed distances from nucleus. Electrons that leave one orbit must move to another orbit. Electrons only change orbits if specific amounts (quanta) of extra energy from the outside world are involved. Electrons that receive enough extra energy from the outside world can leave the atom they are in. Electrons that return to orbits they used to reside in give up the extra energy they acquired when they moved in the first place. Electronic energy given up when electrons move back into an original orbit often shows up as a specific color light. 2

Pauli Exclusion Principle The Exclusion Principle No two electrons in the same atom can have the same set of 4 quantum number values. Wolfgang Ernst Pauli 1900 -1958 Nobel prize in 1945 3

Pauli Exclusion Principle Four Quantum Number Bohr Atom Model n the principal quantum number l the angular momentum (azimuthal) quantum number m the magnetic quantum number s the electron spin quantum number EVERY electron has its own individual set of quantum number values Short hand way to use 4 quantum numbers to describe electron energy levels. The three lowest energy terms (orbits) for a lithium atom. 1, 0, 0, +1/2 1, 0, 0, -1/2 2, 0, 0, +1/2 The short hand notation for lithium atom electron energy levels. 1 s 2 s 4

Pauli Exclusion Principle Atoms with electron configurations that have the lowest energy possible are in their GROUND STATE. The short hand notation for lithium atom electrons in the ground state. 1 s 2 s arrow indicates the electron spin direction. Try three more examples. (1) What is the ground state electron configuration for a nitrogen atom 1 s 2 s 2 p 2 p 2 p x y z (atomic number 7)? (2) What are the lowest energy terms (orbits) for a fluorine atom (atomic number 9)? 1 s 2 s 2 p 2 p 2 p x y z What are the lowest energy terms (orbits) (the ground state) for a neon atom 1 s 2 s 2 p 2 p 2 p x y z (3) (atomic number 10)? 5

Pauli Exclusion Principle The ground state electron configuration for atoms from atomic number 1 (hydrogen) through atomic number 18 (argon) are determined by a repetitive pattern with no two electrons having the same set of quantum numbers. 2 electrons (electron pairs have opposite spins) 1 s 2 electrons 2 s 2 electrons 3 s 6 electrons [2 p 2 p 2 p ] x y z 6 electrons [3 p 3 p 3 p ] x y z 6 A maximum of 9 energy states A maximum of 2 electrons in each energy state

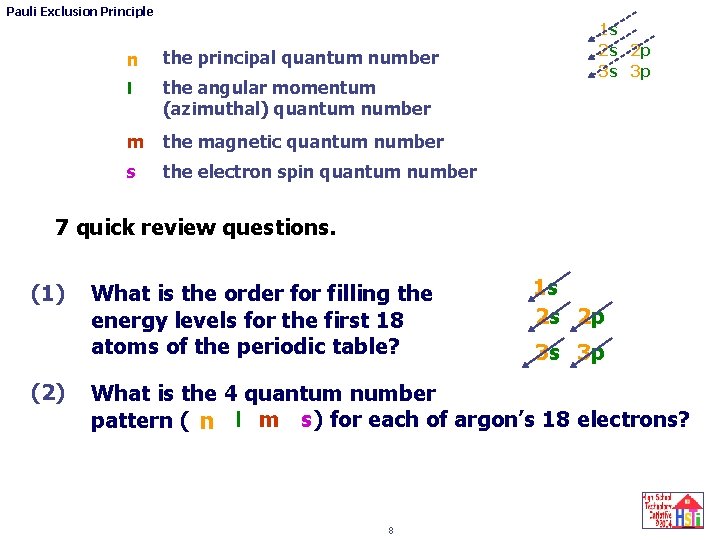
Pauli Exclusion Principle 1 H 3 Li 11 Na 19 K 1 s 2 He 4 Be 12 Mg 6 C 7 N 8 O 9 F 10 Ne 13 Al 14 Si 15 P 16 S 17 Cl 18 Ar Various shorthand ways to describe the ground state electron configuration for beryllium, fluorine and some of the other first 18 atoms in the periodic table are shown below. 1 s 1 s 2 s Ne 3 s Ar 4 s 5 B Ar 4 s Be 2 p 2 p 2 p x y z x 2 2 6 1 s 2 s 2 p Are there other common shorthand notations for Fluorine? 1 s 2 s 2 p x 2 p y 2 p z 7 And/or 1 s 2 2 5 , 2 s , 2 p

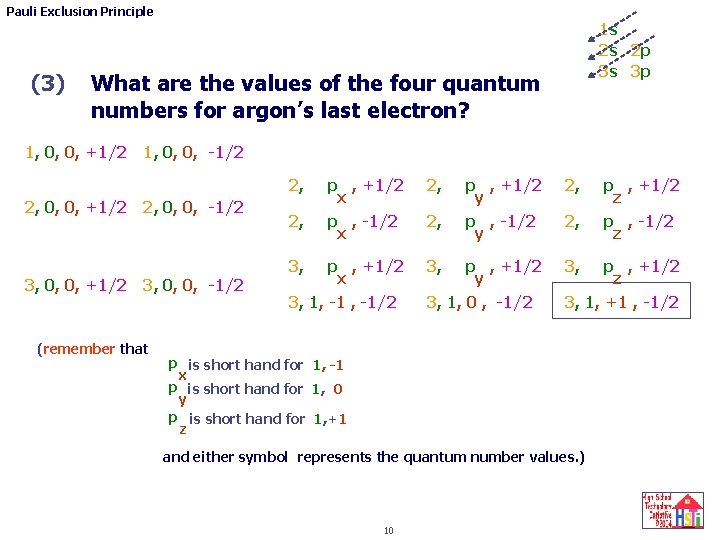
Pauli Exclusion Principle n the principal quantum number l the angular momentum (azimuthal) quantum number m the magnetic quantum number s the electron spin quantum number 1 s 2 s 2 p 3 s 3 p 7 quick review questions. (1) (2) What is the order for filling the energy levels for the first 18 atoms of the periodic table? 1 s 2 s 2 p 3 s 3 p What is the 4 quantum number pattern ( n l m s) for each of argon’s 18 electrons? 8

Pauli Exclusion Principle 1 s 2 s 2 p 3 s 3 p Argon’s 18 electrons 1, 0, 0, +1/2 1, 0, 0, -1/2 2, 2, 0, 0, +1/2 2, 0, 0, -1/2 p , +1/2 x p , -1/2 x 2, p , +1/2 x 3, 1, p -1 , -1/2 x 3, 2, 3, 0, 0, +1/2 3, 0, 0, -1/2 (remember that: p , +1/2 y p , -1/2 y 2, p , +1/2 y 3, 1, p 0 , , -1/2 y 3, 2, p , +1/2 z 3, 1, p +1, , -1/2 z p is short hand for 1, -1 x p is short hand for 1, 0 y p is short hand for 1, +1 z and either symbol represents the quantum number values. 9 p , +1/2 z p , -1/2 z

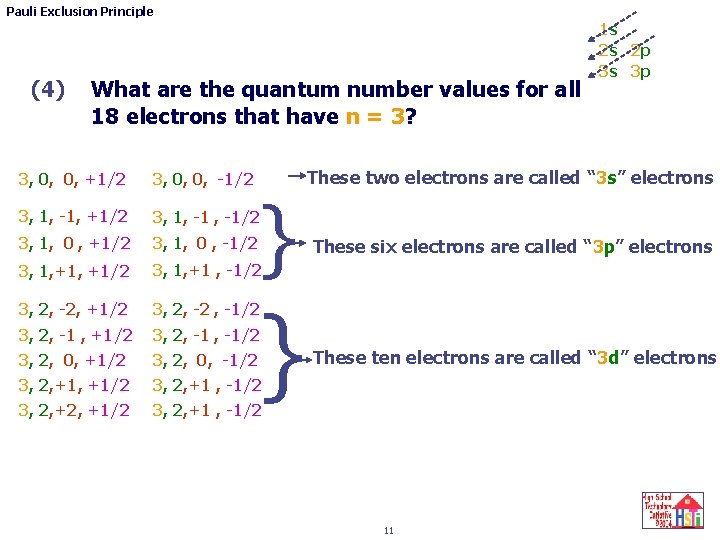
Pauli Exclusion Principle (3) 1 s 2 s 2 p 3 s 3 p What are the values of the four quantum numbers for argon’s last electron? 1, 0, 0, +1/2 1, 0, 0, -1/2 2, 2, 0, 0, +1/2 2, 0, 0, -1/2 p , +1/2 x p , -1/2 x 2, p , +1/2 x 3, 1, -1/2 3, 2, 3, 0, 0, +1/2 3, 0, 0, -1/2 (remember that p , +1/2 y p , -1/2 y 2, p , +1/2 y 3, 1, 0 , -1/2 3, 2, p , +1/2 z 3, 1, +1 , -1/2 p is short hand for 1, -1 x p is short hand for 1, 0 y p is short hand for 1, +1 z and either symbol represents the quantum number values. ) 10 p , +1/2 z p , -1/2 z

Pauli Exclusion Principle (4) What are the quantum number values for all 18 electrons that have n = 3? 3, 0, 0, +1/2 3, 0, 0, -1/2 3, 1, -1, +1/2 3, 1, -1/2 3, 1, 0 , +1/2 3, 1, 0 , -1/2 3, 1, +1/2 3, 1, +1 , -1/2 3, 2, -2, +1/2 3, 2, -2 , -1/2 3, 2, -1 , +1/2 3, 2, -1/2 3, 2, 0, +1/2 3, 2, 0, -1/2 3, 2, +1/2 3, 2, +1 , -1/2 } } 1 s 2 s 2 p 3 s 3 p These two electrons are called “ 3 s” electrons These six electrons are called “ 3 p” electrons These ten electrons are called “ 3 d” electrons 11

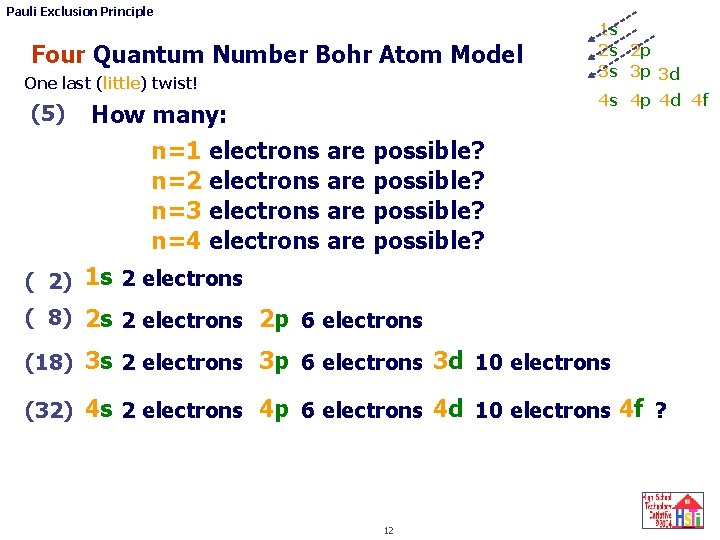
Pauli Exclusion Principle Four Quantum Number Bohr Atom Model One last (little) twist! (5) 1 s 2 s 2 p 3 s 3 p 3 d 4 s 4 p 4 d 4 f How many: n=1 electrons are possible? n=2 electrons are possible? n=3 electrons are possible? n=4 electrons are possible? ( 2) 1 s 2 electrons ( 8) 2 s 2 electrons 2 p 6 electrons (18) 3 s 2 electrons 3 p 6 electrons 3 d 10 electrons (32) 4 s 2 electrons 4 p 6 electrons 4 d 10 electrons 4 f ? 12

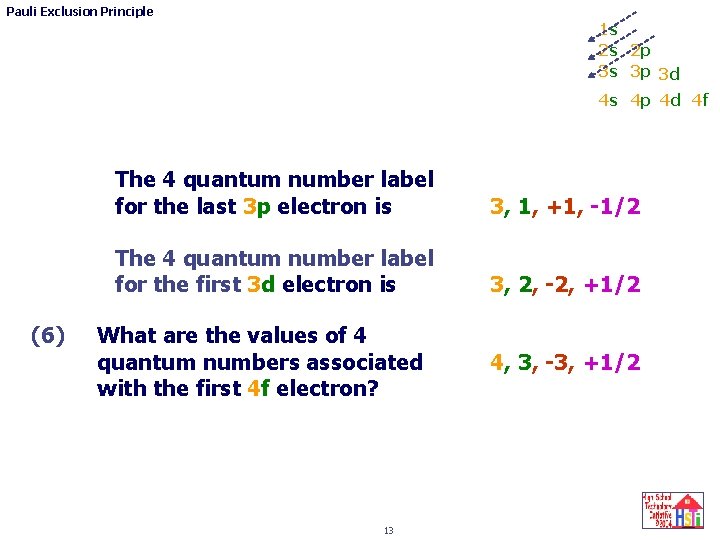
Pauli Exclusion Principle 1 s 2 s 2 p 3 s 3 p 3 d 4 s 4 p 4 d 4 f (6) The 4 quantum number label for the last 3 p electron is 3, 1, +1, -1/2 The 4 quantum number label for the first 3 d electron is 3, 2, -2, +1/2 What are the values of 4 quantum numbers associated with the first 4 f electron? 13 4, 3, -3, +1/2

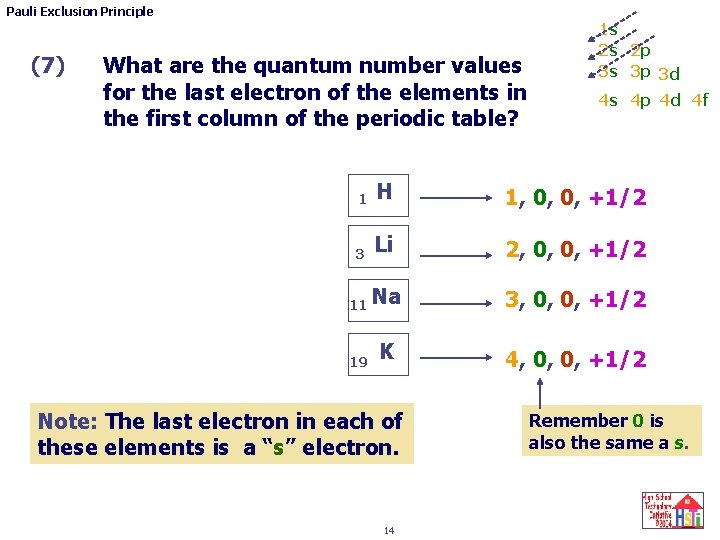
Pauli Exclusion Principle (7) What are the quantum number values for the last electron of the elements in the first column of the periodic table? 1 s 2 s 2 p 3 s 3 p 3 d 4 s 4 p 4 d 4 f 1 H 1, 0, 0, +1/2 3 Li 2, 0, 0, +1/2 11 Na 19 K Note: The last electron in each of these elements is a “s” electron. 14 3, 0, 0, +1/2 4, 0, 0, +1/2 Remember 0 is also the same a s.

Pauli Exclusion Principle 15