Pauli Exclusion principle Definition No two electrons can




- Slides: 9

Pauli – Exclusion principle • Definition: No two electrons can have the same four quantum numbers, or no two electrons can occupy the exact same energy state. • Contribution to atomic model: Explains why electrons in atoms are arranged in their orbitals as they are. • First orbital (1 S) only 2 electrons • Second orbital (2 S and P) contains 8 • • Principle quantum number (n) - related to Bohr’s energy shells Orbital Quantum number (l) – shape and angular momentum of orbital Magnetic Quantum number (ml ) – Magnetic orientation of electron Magnetic Spin Quantum number (ms) – Electron spin around own axis

Schrödinger Wave function and onwards

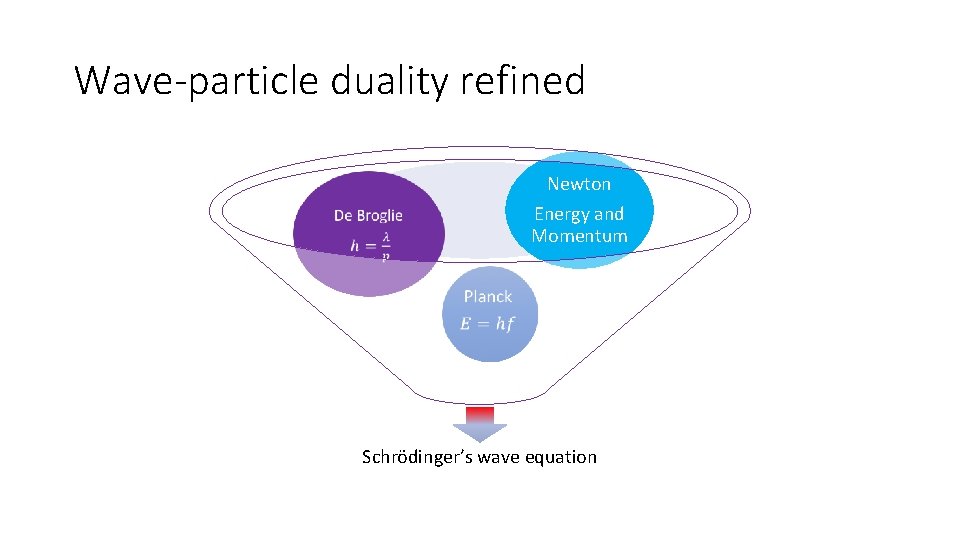
Wave-particle duality refined Newton Energy and Momentum Schrödinger’s wave equation

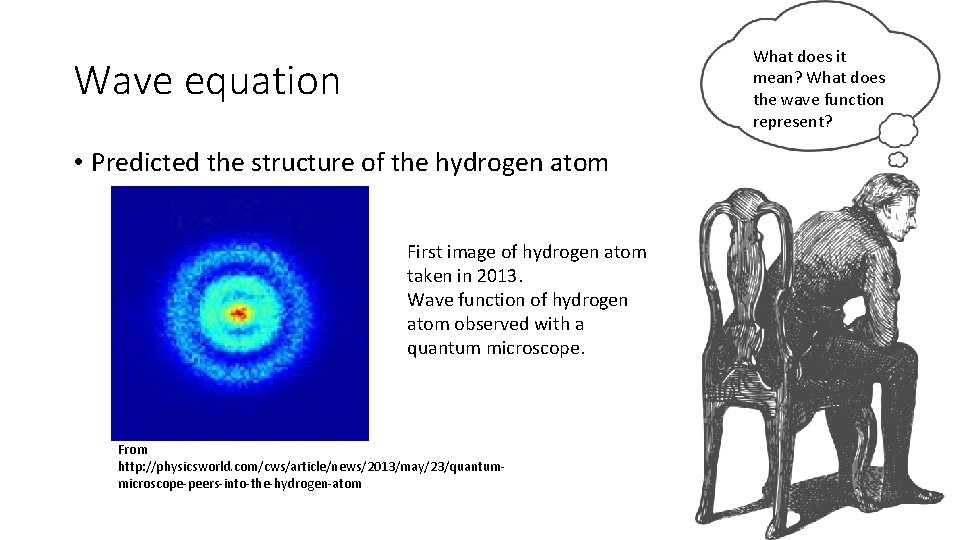
What does it mean? What does the wave function represent? Wave equation • Predicted the structure of the hydrogen atom First image of hydrogen atom taken in 2013. Wave function of hydrogen atom observed with a quantum microscope. From http: //physicsworld. com/cws/article/news/2013/may/23/quantummicroscope-peers-into-the-hydrogen-atom

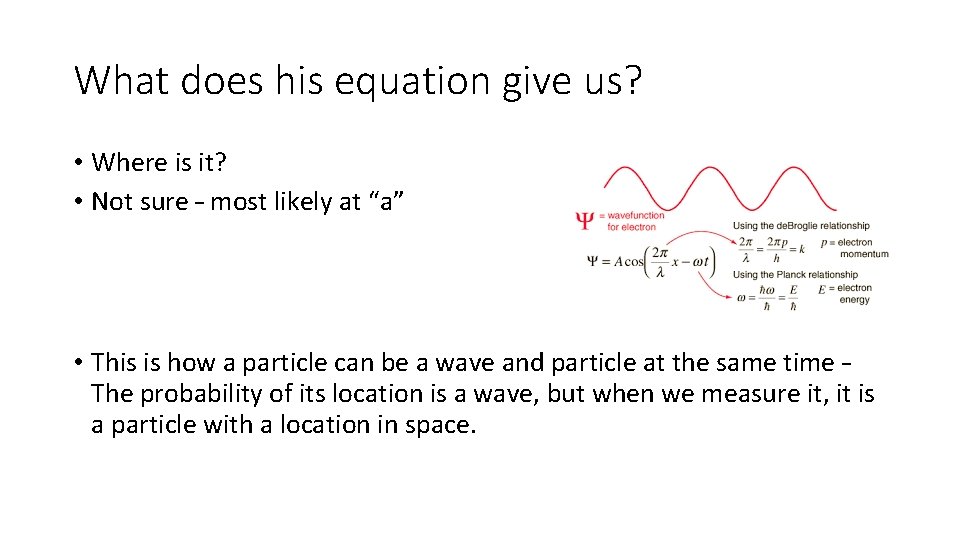
What does his equation give us? • Where is it? • Not sure – most likely at “a” • This is how a particle can be a wave and particle at the same time – The probability of its location is a wave, but when we measure it, it is a particle with a location in space.

• Schrödinger proposed that there is a wave associated with any particle (like the electron), called the wavefunction. • It is spread out to fill the whole universe. The wave function is stronger in a region, which corresponds to the position of the particle and gets weaker farther away from this region but still exists even far away from the "position" of the particle. • He formulated the Schrödinger equation which is very good at predicting how particles like electrons behave under different circumstances.

Heisenberg – Uncertainty Principle • Definition: Product of the uncertainty in measuring position and uncertainty in measuring Momentum is always equal or larger than a constant. • Contribution: Heisenberg used the concept of a wavefunction to prove that nature does not allow us to measure the position and velocity of a single particle (let alone the whole universe) with perfection no matter how precise our measuring instruments. • Thus the more accurately you try to measure the position of the particle, the less accurately you can measure its speed, and vice versa. • This finding is known as an uncertainty principle

Practise Question • Analyse the concept of quantum mechanics in terms of its probabilistic nature and contrast it to the deterministic nature of classical physics.

Sample Answer •