Patterns of Chemical Reactivity Periodic Table predicts the
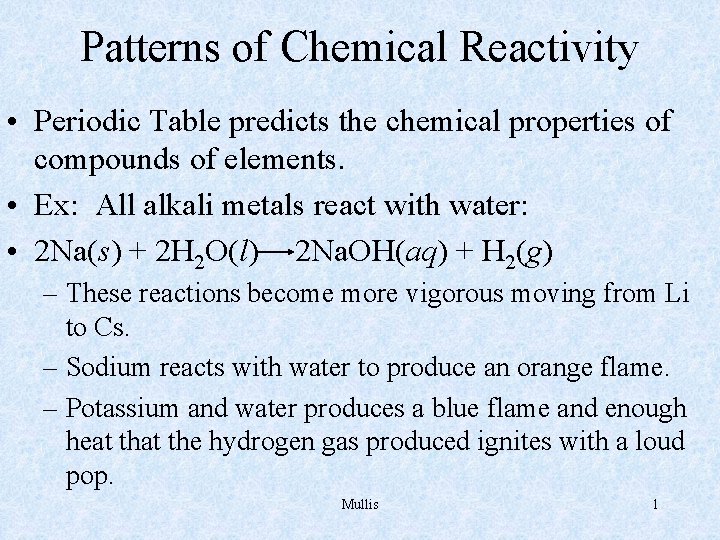
Patterns of Chemical Reactivity • Periodic Table predicts the chemical properties of compounds of elements. • Ex: All alkali metals react with water: • 2 Na(s) + 2 H 2 O(l) 2 Na. OH(aq) + H 2(g) – These reactions become more vigorous moving from Li to Cs. – Sodium reacts with water to produce an orange flame. – Potassium and water produces a blue flame and enough heat the hydrogen gas produced ignites with a loud pop. Mullis 1
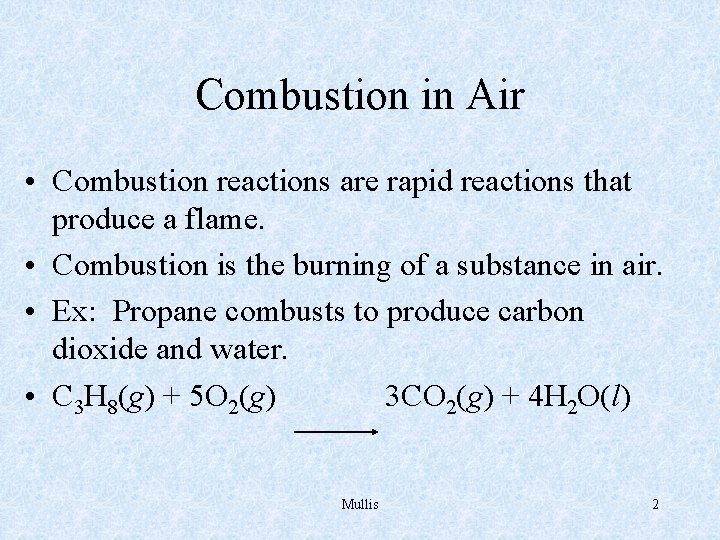
Combustion in Air • Combustion reactions are rapid reactions that produce a flame. • Combustion is the burning of a substance in air. • Ex: Propane combusts to produce carbon dioxide and water. • C 3 H 8(g) + 5 O 2(g) 3 CO 2(g) + 4 H 2 O(l) Mullis 2
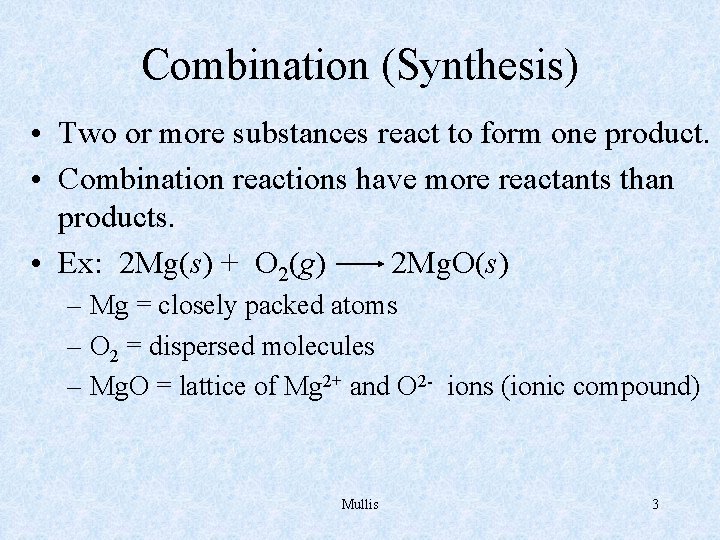
Combination (Synthesis) • Two or more substances react to form one product. • Combination reactions have more reactants than products. • Ex: 2 Mg(s) + O 2(g) 2 Mg. O(s) – Mg = closely packed atoms – O 2 = dispersed molecules – Mg. O = lattice of Mg 2+ and O 2 - ions (ionic compound) Mullis 3
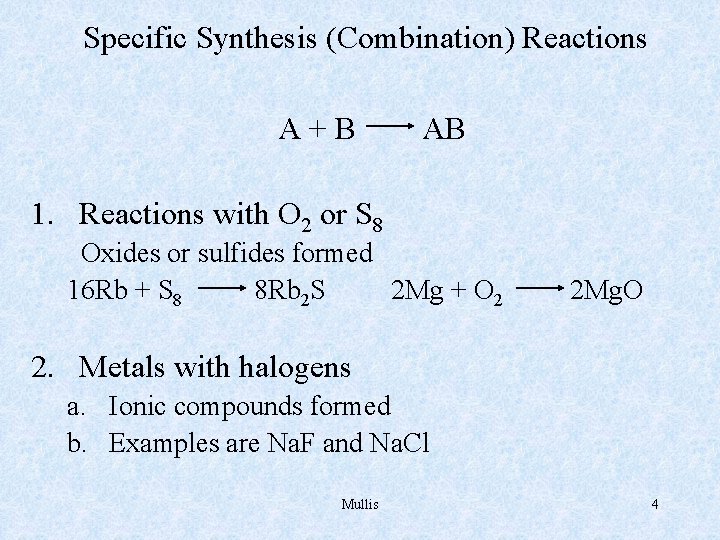
Specific Synthesis (Combination) Reactions A+B AB 1. Reactions with O 2 or S 8 Oxides or sulfides formed 16 Rb + S 8 8 Rb 2 S 2 Mg + O 2 2 Mg. O 2. Metals with halogens a. Ionic compounds formed b. Examples are Na. F and Na. Cl Mullis 4

Synthesis reactions, cont. A+B AB 3. Two compounds combine to form a single product. 4. N 2 O 3 + H 2 O 2 HNO 2 4. Oxides with water a. With metal: Metal hydroxides produced Ca. O + H 2 O Ca(OH)2 b. With nonmetal: Oxyacids produced SO 3 + H 2 O H 2 SO 4 Mullis 5
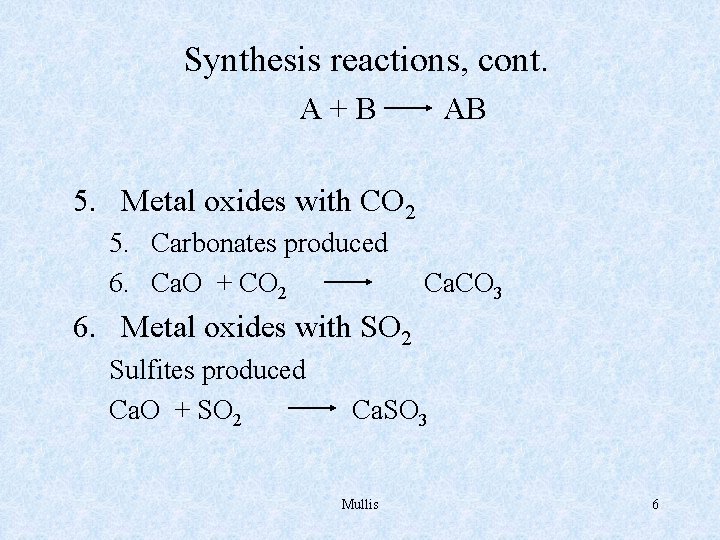
Synthesis reactions, cont. A+B AB 5. Metal oxides with CO 2 5. Carbonates produced 6. Ca. O + CO 2 Ca. CO 3 6. Metal oxides with SO 2 Sulfites produced Ca. O + SO 2 Ca. SO 3 Mullis 6
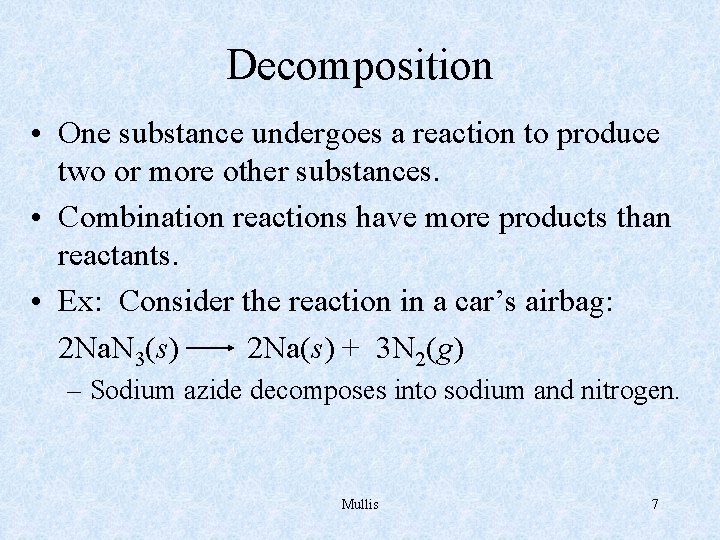
Decomposition • One substance undergoes a reaction to produce two or more other substances. • Combination reactions have more products than reactants. • Ex: Consider the reaction in a car’s airbag: 2 Na. N 3(s) 2 Na(s) + 3 N 2(g) – Sodium azide decomposes into sodium and nitrogen. Mullis 7
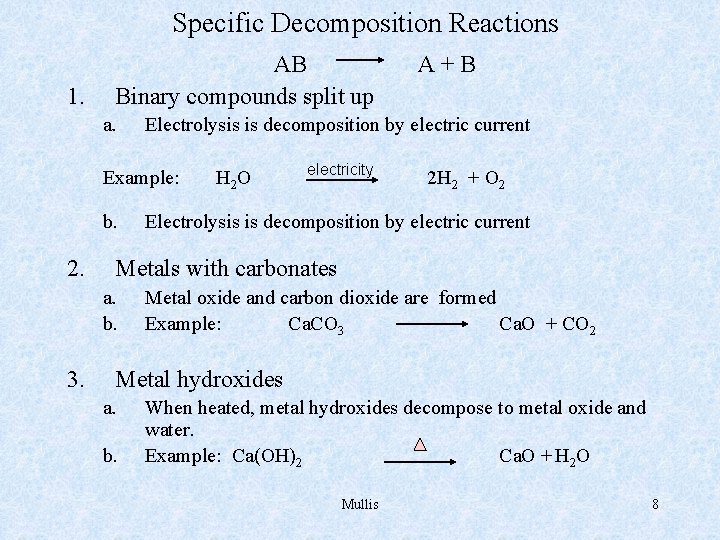
Specific Decomposition Reactions 1. AB Binary compounds split up a. Electrolysis is decomposition by electric current Example: b. 2. H 2 O electricity 2 H 2 + O 2 Electrolysis is decomposition by electric current Metals with carbonates a. b. 3. A+B Metal oxide and carbon dioxide are formed Example: Ca. CO 3 Ca. O + CO 2 Metal hydroxides a. b. When heated, metal hydroxides decompose to metal oxide and water. Example: Ca(OH)2 Ca. O + H 2 O Mullis 8
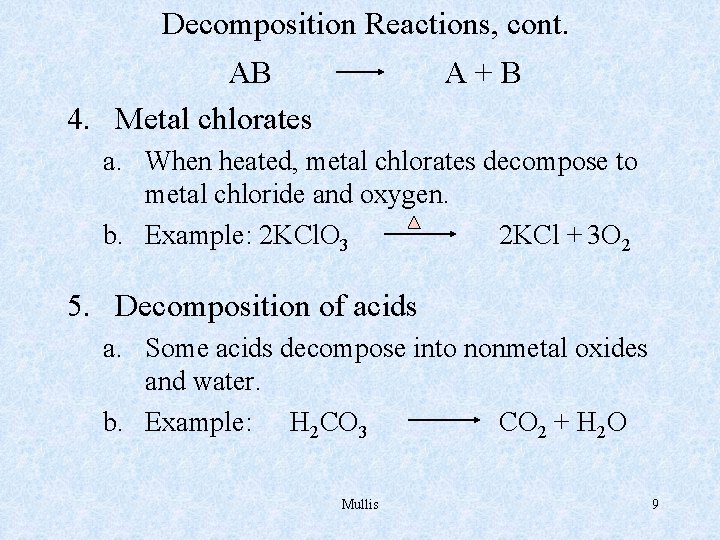
Decomposition Reactions, cont. AB 4. Metal chlorates A+B a. When heated, metal chlorates decompose to metal chloride and oxygen. b. Example: 2 KCl. O 3 2 KCl + 3 O 2 5. Decomposition of acids a. Some acids decompose into nonmetal oxides and water. b. Example: H 2 CO 3 CO 2 + H 2 O Mullis 9
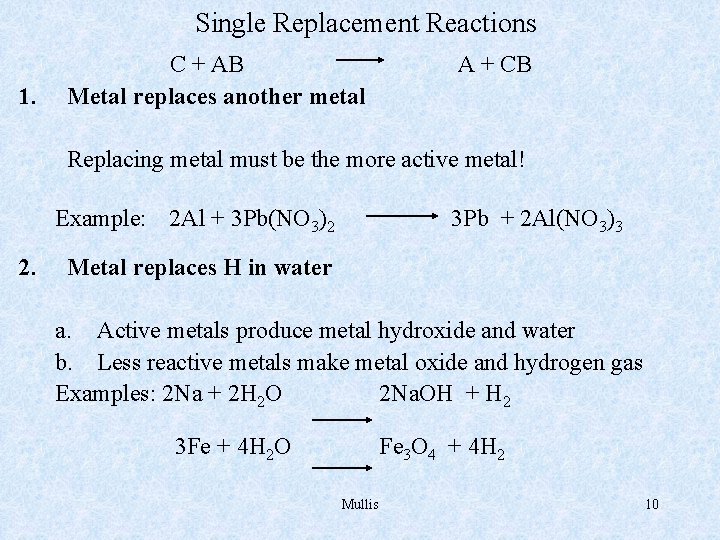
Single Replacement Reactions 1. C + AB Metal replaces another metal A + CB Replacing metal must be the more active metal! Example: 2 Al + 3 Pb(NO 3)2 2. 3 Pb + 2 Al(NO 3)3 Metal replaces H in water a. Active metals produce metal hydroxide and water b. Less reactive metals make metal oxide and hydrogen gas Examples: 2 Na + 2 H 2 O 2 Na. OH + H 2 3 Fe + 4 H 2 O Fe 3 O 4 + 4 H 2 Mullis 10
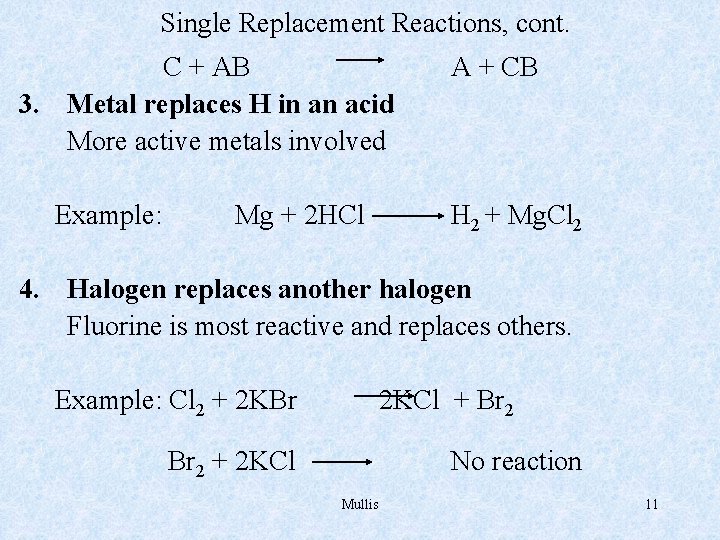
Single Replacement Reactions, cont. C + AB 3. Metal replaces H in an acid More active metals involved Example: Mg + 2 HCl A + CB H 2 + Mg. Cl 2 4. Halogen replaces another halogen Fluorine is most reactive and replaces others. Example: Cl 2 + 2 KBr 2 KCl + Br 2 + 2 KCl No reaction Mullis 11
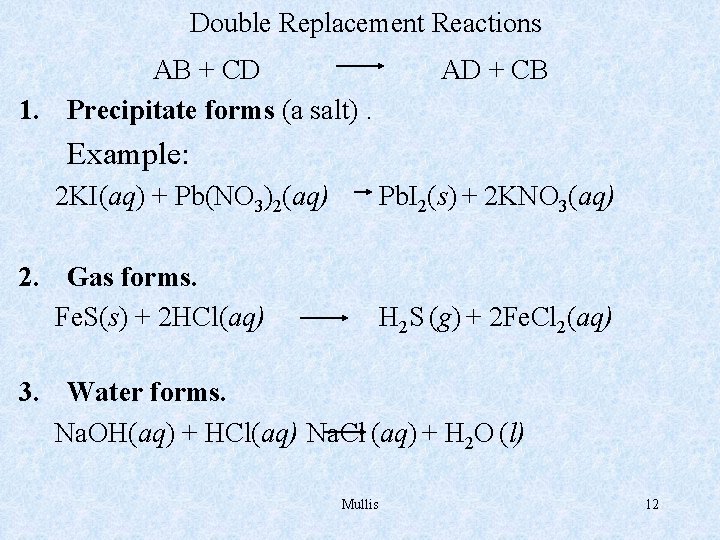
Double Replacement Reactions AB + CD 1. Precipitate forms (a salt). AD + CB Example: 2 KI(aq) + Pb(NO 3)2(aq) Pb. I 2(s) + 2 KNO 3(aq) 2. Gas forms. Fe. S(s) + 2 HCl(aq) H 2 S (g) + 2 Fe. Cl 2(aq) 3. Water forms. Na. OH(aq) + HCl(aq) Na. Cl (aq) + H 2 O (l) Mullis 12
- Slides: 12