Patterns in the Periodic Table 6 4 Chemical

Patterns in the Periodic Table 6. 4

Chemical Families • CHEMICAL FAMILY: a column of elements on the periodic table with similar physical and chemical properties • Each family has it’s own name • Columns are numbered left to right

What are the families? • ALKALI METAL: element in group 1 of the periodic table • Shiny, silvery, and soft • Highly reactive, so often combine with other elements and compounds • Examples: Salt (Na. Cl), baking soda (Na. HCO 3), potassium (bananas!)

What are the families? • ALKALINE EARTH METAL: an element in group 2 of the periodic table • Shiny, silvery, not as soft as group 1 • Not as reactive as group 1 • Examples: calcium (milk!), fireworks (bright flames)

What are the families? • NOBLE GASES: element in group 18 of the periodic table • Colourless, tasteless, odourless • Unreactive • Non-toxic (except for radon) • The diff noble gases glow diff colours when an electrical current passes through them • Examples: Helium (balloons), Neon (signs)
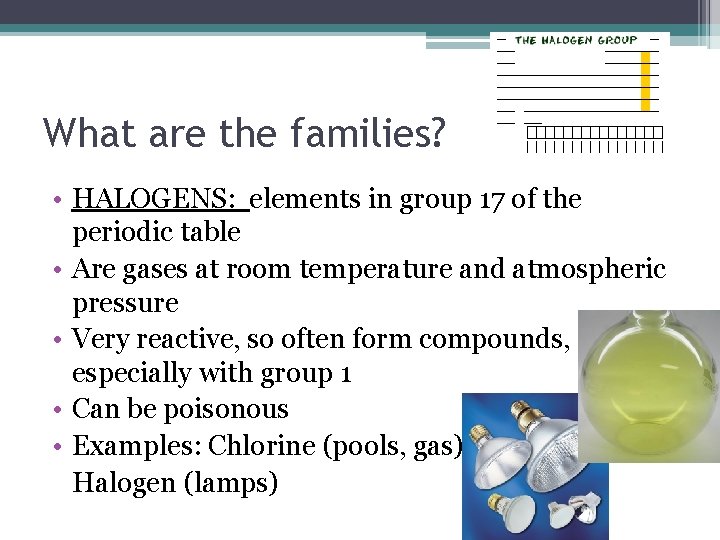
What are the families? • HALOGENS: elements in group 17 of the periodic table • Are gases at room temperature and atmospheric pressure • Very reactive, so often form compounds, especially with group 1 • Can be poisonous • Examples: Chlorine (pools, gas), Halogen (lamps)

Periodic Trends • Elements in the same row also show trends • PERIOD: a row in the periodic table • Reactivity: group 1 more reactive than group 2, group 17 more reactive than group 16 Now let’s look at some elements!

Homework Questions • Pg. 225 #1, 2, 8, 9, 10 • #7 (done as a class), we will colour in periodic tables!
- Slides: 8