PatientReported Outcome Measures Use in Medical Product Development







- Slides: 10

Patient-Reported Outcome Measures: Use in Medical Product Development to Support Labeling Claims US FDA

Introduction • FDA issued its Guidance for Industry on the review and evaluation of Patient Reported Outcomes (PROs) in support of labeling claims. – Specifically, it addresses issues surrounding PRO instruments (surveys) that measure treatment benefits measured in clinical trials. – The guidance does not address disease- specific issues. – The guidance stresses the need to meet with the FDA prior to initiating a study so that a consensus can be reached with respect to the instrument.

Background • PRO – any health status report coming directly from the patient – Outcomes measured in absolute terms (severity of a symptom, sign, or state of disease) – In clinical trials, the PRO can be used to measure the effect of the intervention on one or more concepts.

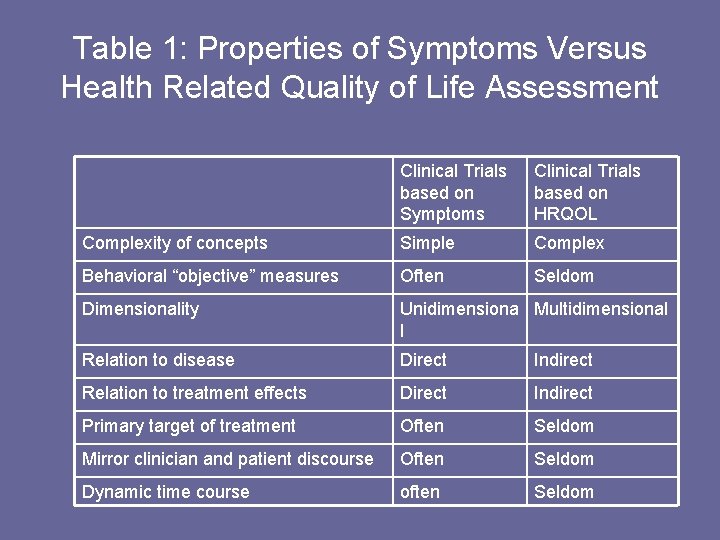
Table 1: Properties of Symptoms Versus Health Related Quality of Life Assessment Clinical Trials based on Symptoms Clinical Trials based on HRQOL Complexity of concepts Simple Complex Behavioral “objective” measures Often Seldom Dimensionality Unidimensiona Multidimensional l Relation to disease Direct Indirect Relation to treatment effects Direct Indirect Primary target of treatment Often Seldom Mirror clinician and patient discourse Often Seldom Dynamic time course often Seldom

PRO Acceptability • Document the history and psychometric properties (reliability and validity) for the instrument. • Modified instruments must define the degree of modification: – Decide how much revalidation is necessary – Provide justification to the FDA for the modification.

Evaluation of a PRO Instrument • • The population enrolled in the clinical trial. The clinical trial objectives and design. The PRO instrument’s conceptual framework. The PRO instrument’s measurement properties.

How will PRO data be used? • Findings measured by a well-defined and reliable PRO instrument can be used to support a claim in medical product labeling. • Claims generally appear in either the Indications and Usage or Clinical Studies section of the label, but can appear in any section.

Documentation for instruments • Conceptual framework – research must clearly define what is being measured, how it will be measured, and why it is being measured (what is the label claim being supported). • Administrative characteristics – What will the instrument look like when it is administered, for instance, the test format, patient instructions, test items, response option, data collection method, and scoring procedures. • Performance characteristics – reliability and validity must be demonstrated for the FDA. • Study design – need to discuss with the FDA how the instrument fits within the study design and how the study design will adequately and accurately evaluate the primary hypothesis for the study.

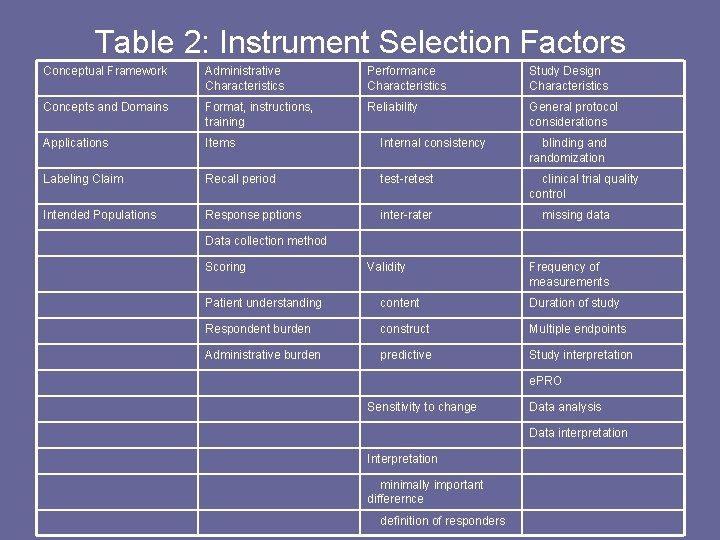
Table 2: Instrument Selection Factors Conceptual Framework Administrative Characteristics Performance Characteristics Study Design Characteristics Concepts and Domains Format, instructions, training Reliability General protocol considerations Applications Items Internal consistency blinding and randomization Labeling Claim Recall period test-retest clinical trial quality control Intended Populations Response pptions inter-rater missing data Data collection method Scoring Validity Frequency of measurements Patient understanding content Duration of study Respondent burden construct Multiple endpoints Administrative burden predictive Study interpretation e. PRO Sensitivity to change Data analysis Data interpretation Interpretation minimally important differernce definition of responders

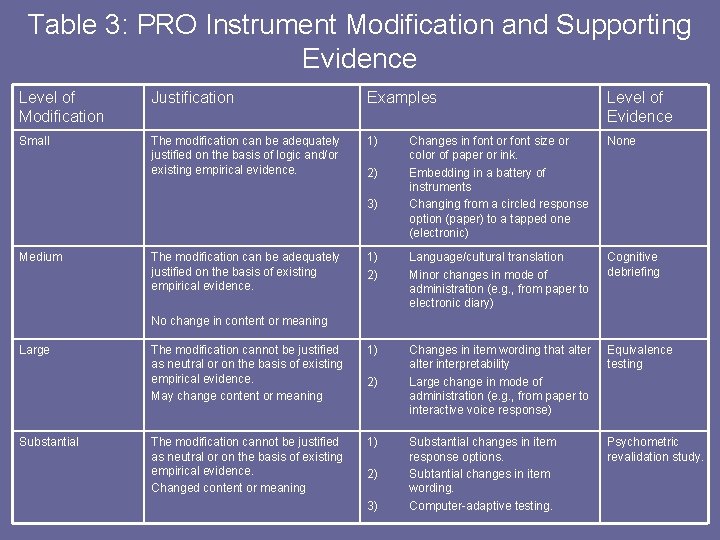
Table 3: PRO Instrument Modification and Supporting Evidence Level of Modification Justification Examples Level of Evidence Small The modification can be adequately justified on the basis of logic and/or existing empirical evidence. 1) Changes in font or font size or color of paper or ink. Embedding in a battery of instruments Changing from a circled response option (paper) to a tapped one (electronic) None 1) 2) Language/cultural translation Minor changes in mode of administration (e. g. , from paper to electronic diary) Cognitive debriefing The modification cannot be justified as neutral or on the basis of existing empirical evidence. May change content or meaning 1) Changes in item wording that alter interpretability Large change in mode of administration (e. g. , from paper to interactive voice response) Equivalence testing The modification cannot be justified as neutral or on the basis of existing empirical evidence. Changed content or meaning 1) Substantial changes in item response options. Subtantial changes in item wording. Computer-adaptive testing. Psychometric revalidation study. 2) 3) Medium The modification can be adequately justified on the basis of existing empirical evidence. No change in content or meaning Large Substantial 2) 2) 3)