Patient Engagement in Drug Development Experiences Good Practices



![“When it comes to terminal illnesses [the FDA’s] job should be to make sure “When it comes to terminal illnesses [the FDA’s] job should be to make sure](https://slidetodoc.com/presentation_image_h/29a81416f3e5a1479f01db3bd49df176/image-8.jpg)







- Slides: 15

Patient Engagement in Drug Development: Experiences, Good Practices, Lessons Learned Parent. Project. MD. org

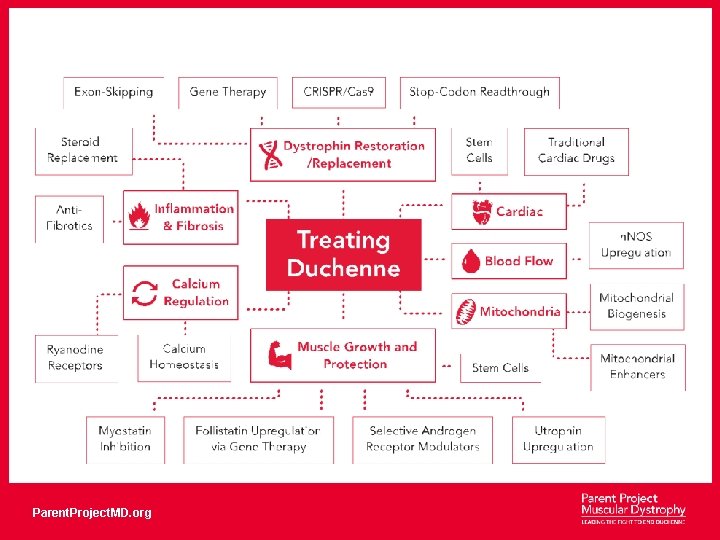
About Duchenne muscular dystrophy X-linked, pediatric neuromuscular disease, with onset in early childhood Incidence rate: 1: 4600 boys (30% spontaneous) Diagnosis: 3 -5 years of age Predictable course Progressive loss of function 100% lethal Parent. Project. MD. org

Parent. Project. MD. org

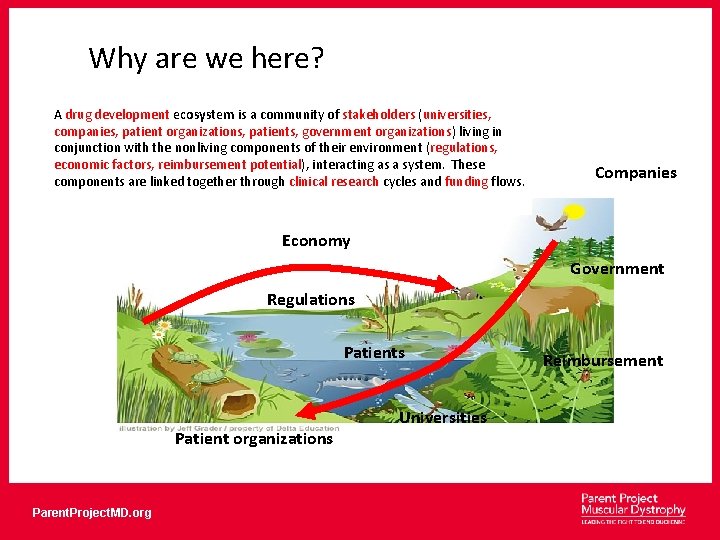
Why are we here? A drug development ecosystem is a community of stakeholders (universities, companies, patient organizations, patients, government organizations) living in conjunction with the nonliving components of their environment (regulations, economic factors, reimbursement potential), interacting as a system. These components are linked together through clinical research cycles and funding flows. Companies Economy Government Regulations Patient organizations Parent. Project. MD. org Universities Reimbursement

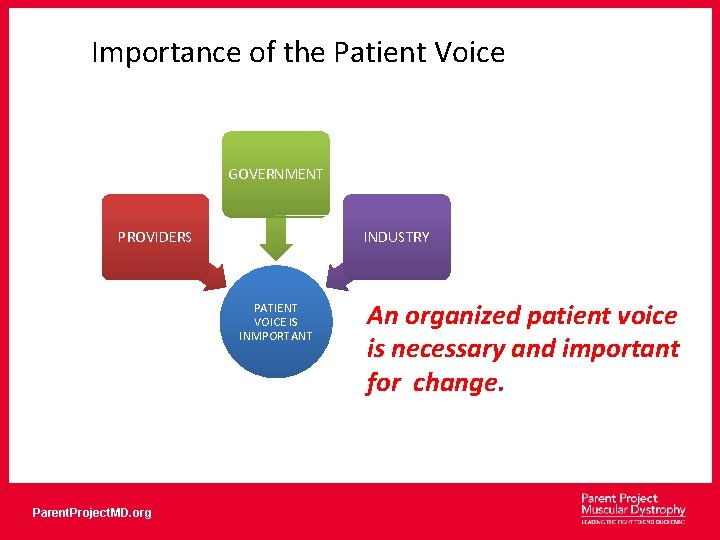
Importance of the Patient Voice GOVERNMENT PROVIDERS INDUSTRY PATIENT VOICE IS INMPORTANT Parent. Project. MD. org An organized patient voice is necessary and important for change.

Regulatory Journey Policy Partnership Include the Patient Voice BUT – Request Transparency from Regulatory Agencies • Understand What was Included into the Decision Making Process • • • – Patient Reported Outcomes (PRO) – Preference Data Parent. Project. MD. org

PPMD has been advocating for methods to assess the benefitsrisks of treatments for rare disease. We have also: • Validated this work • Studied caregiver worries • Published a white paper on benefit risk Parent. Project. MD. org 7
![When it comes to terminal illnesses the FDAs job should be to make sure “When it comes to terminal illnesses [the FDA’s] job should be to make sure](https://slidetodoc.com/presentation_image_h/29a81416f3e5a1479f01db3bd49df176/image-8.jpg)
“When it comes to terminal illnesses [the FDA’s] job should be to make sure a product is safe and that the risks and benefits presented by the producer are accurate. Our job should be to determine, given all that information, whether to give it to our children. It is an intensely personal decision that involves the parents and the child with Duchenne. ” Parent. Project. MD. org

WORKING with BIO Parent. Project. MD. org 9

FDA Engagement – A collaborative Community • • Meetings with Division of Neurology Duchenne Policy Forum (December 2013) PPMD submits Draft Guidance (June, 2014) NIH/FDA/PPMD meeting –dystrophin quantification methodologies (February, 2015) • FDA releases Draft Guidance (June, 2015) Setting the stage for the draft guidance Parent. Project. MD. org

Clinical Trial, NDA, Ad. Comm • flawed study – Outcomes, dystrophin, open label, small number – Scientific community support – 1000 people participated in the Advisory Committee – 52 speakers only 1 negative – Advisory Committee Split Vote – PDUFA Date ignored Parent. Project. MD. org

The Decision • September 19 2016 – Eteplirsen Approved (Exondys 51) Parent. Project. MD. org

The Fallout • 162 page FDA document describes agency turmoil • NORD meeting Dr. Jenkins states ‘eteplirsen should not be approved’ Parent. Project. MD. org Sarepta Therapeutics Announces Third Quarter 2016 Financial Results and … Business Wire 15 h Humana spells out its conditional Exondys 51 coverage policy — … endpts. com 1 d Sarepta Therapeutics (SRPT) Stock: They Can Shake And Rattle, But Will … cnafinance. com 1 d After 'Female Viagra, ' Muscular Dystrophy Drug, Will FDA Stand … Forbes 1 d The FDA’s Controversial Duchenne Drug Approval And The Moral … Health Affairs 2 d FDA expert lashes out at 'worrisome' Sarepta approval in JAMA Fierce Biotech 2 d

Learnings • Rare Disease is hard • Deserves the greatest degree of flexibility when making decisions • Once a drug is approved, utilizing the established process – ALL STAKEHOLDERS MUST MAKE PEACE WITH THE DECISION • Disruption encourages conversation. • No one wants their child to receive weekly infusions, injections or for that matter oral drugs if they have no efficacy • Given the trajectory of the illness, the limited life span, once safety is established and a trend toward benefit, consider adaptive licensing. • Without this conversation, patients will wait and wait…. • Eteplirsen is approved – the Community is interested in a real-world experience to fully understand both benefit and risk. Parent. Project. MD. org

The most important piece of the puzzle for developing therapies is the question of MEANINGFUL BENEFIT. THANK YOU! Parent. Project. MD. org