Patient Engagement in Data Sharing Case Study Example

















- Slides: 32

Patient Engagement in Data Sharing Case Study Example

The following information has been developed by: Disclosure Joel Beetsch, Ph. D – VP of Global Patient Advocacy, Celgene Corporation; member of the BD 4 P Steering Committee. Jennifer King, Ph. D – Director of Science and Research, Lung Cancer Alliance; Co-Founder, SHARE For Cures; member of the BD 4 P Steering Committee. This information does not constitute an endorsement by the Celgene Corporation, the Lung Cancer Alliance, Project Data Sphere, or SHARE For Cures.

Patient Engagement in Data Sharing: Patient. Directed Data Sharing & Sharing Clinical Trial Data Compare and contrast: Case Study Opt-in research Data sharing Data privacy Patient data access Focus: Project Data Sphere & SHARE For Cures

Cancer research is slow and expensive It takes up to $2 billion to bring a new drug to market – but an estimated 7. 6 million people die from cancer each year* Cancer Research Overall cancer death rates continue to decline, but progress is far too slow Cancer accounts for more deaths than heart disease We haven’t come far since the “War on Cancer” was declared *Source: Journal of the National Cancer Institute (JNCI)

A free digital library-laboratory that provides one place where the research community can share, integrate, and analyze patient-level data from academic and industry phase III cancer clinical trials Project Data Sphere Available to independent and affiliated researchers – anyone can apply to become an authorized user Fully-public platform of de-identified, historic, raw cancer trial data, protocols and case report form with advanced analytics, and collaboration tools To responsibly speed cancer research What if we could share, integrate, and analyze our collective historical cancer research data in a single location?

Each data provider enters into a Data Sharing Agreement for the data sets provided Data Sharing There is no general consent for individuals to contribute their data The Clinical Data Interchange Standards Consortium (CDISC) Study Data Tabulation Model (SDTM) is the recommended format for data sharing

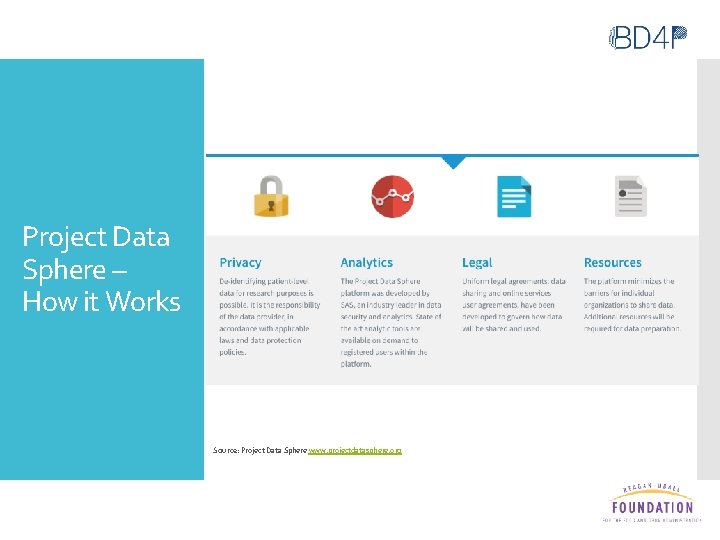
Project Data Sphere – How it Works Source: Project Data Sphere www. projectdatasphere. org

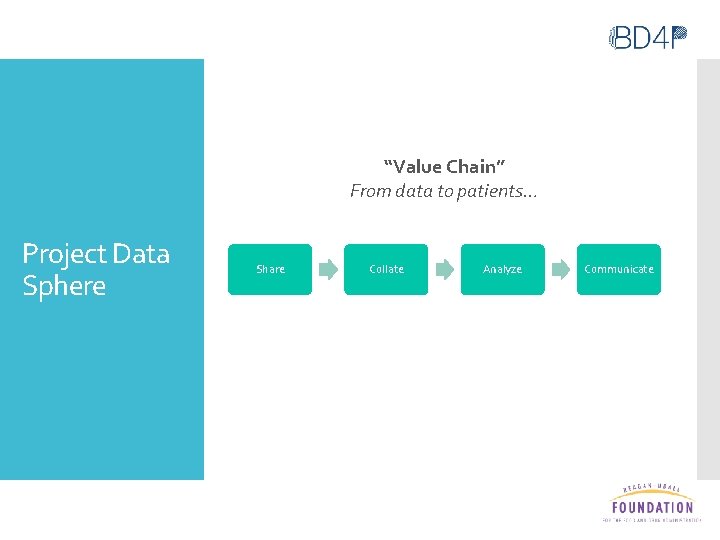
“Value Chain” From data to patients… Project Data Sphere Share Collate Analyze Communicate

7. 6 million lives lost each year worldwide Data-Sharing in Oncology: Why do it…? 1 1) Faster, more efficient research 2) Reduced duplication and transparency 3) Real-world corroboration with trial data 4) Data standards and meta-analysis 5) Unknowns 2 Improved trial design and statistical methodology Secondary hypotheses and epidemiology Disease model development Smaller trials sizing 1. Vickers 2006 2. www. cardia. dopm. uab. edu: 475 publications from a single large dataset

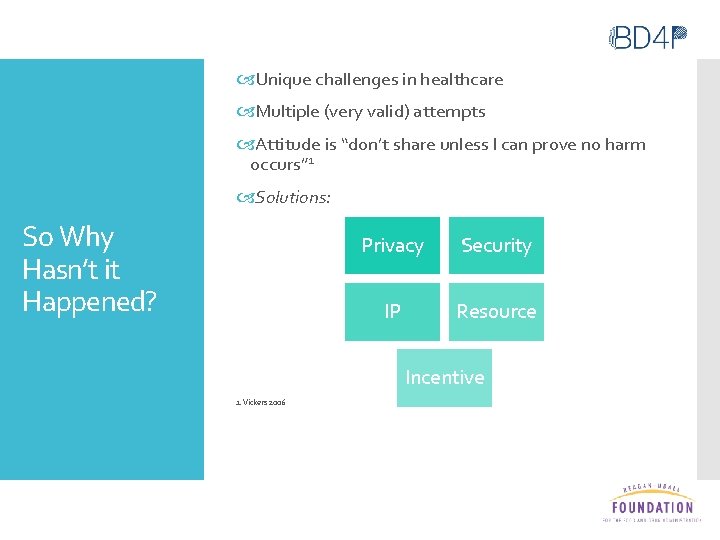
Unique challenges in healthcare Multiple (very valid) attempts Attitude is “don’t share unless I can prove no harm occurs” 1 Solutions: So Why Hasn’t it Happened? Privacy Security IP Resource Incentive 1. Vickers 2006

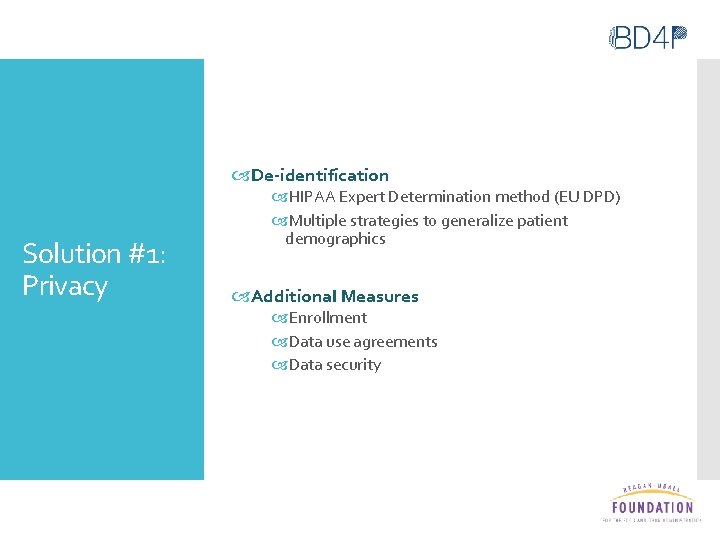
De-identification Solution #1: Privacy HIPAA Expert Determination method (EU DPD) Multiple strategies to generalize patient demographics Additional Measures Enrollment Data use agreements Data security

Hardened SAS Hosting Environment Firewall All access to data behind SAS firewall Secure Socket Layer Transmission Solution #2: Security All transmissions encrypted Content virus scanning All documents scanned before being made public Enrollment Role-based permissions Password policy – to minimize chances of unauthorized entry Application acceptance for access – BUT broad criteria

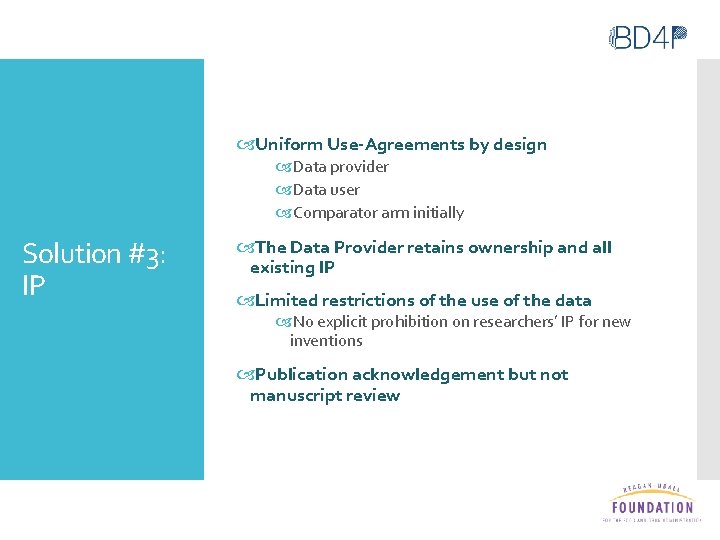
Uniform Use-Agreements by design Data provider Data user Comparator arm initially Solution #3: IP The Data Provider retains ownership and all existing IP Limited restrictions of the use of the data No explicit prohibition on researchers’ IP for new inventions Publication acknowledgement but not manuscript review

Minimal resources required Solution #4: Resources Especially relative to trial cost and benefits of sharing IT, legal, and Project Data Sphere infrastructure in place – no cost Only requirements “Champion” within organization Data preparation i. e. Bio-statistician time to de-identify single Phase III data set (~40 hours of programming) Legal review

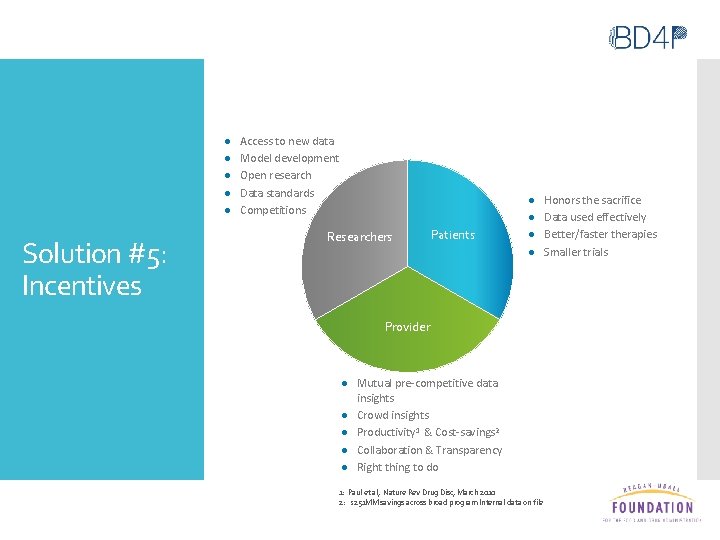
● ● ● Solution #5: Incentives Access to new data Model development Open research Data standards Competitions Researchers Patients ● ● Provider ● Mutual pre-competitive data insights ● Crowd insights ● Productivity 1 & Cost-savings 2 ● Collaboration & Transparency ● Right thing to do 1: Paul et al, Nature Rev Drug Disc, March 2010 2: $251 MM savings across broad program Internal data on file Honors the sacrifice Data used effectively Better/faster therapies Smaller trials

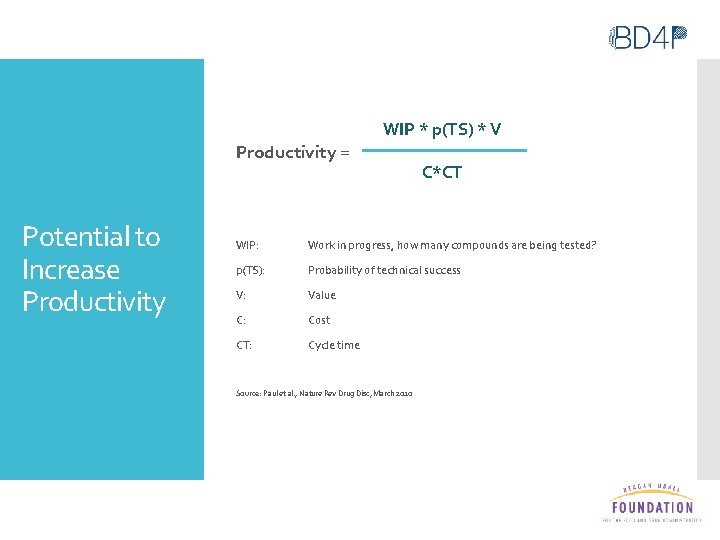
Productivity = Potential to Increase Productivity WIP * p(TS) * V C*CT WIP: Work in progress, how many compounds are being tested? p(TS): Probability of technical success V: Value C: Cost CT: Cycle time Source: Paul et al. , Nature Rev Drug Disc, March 2010

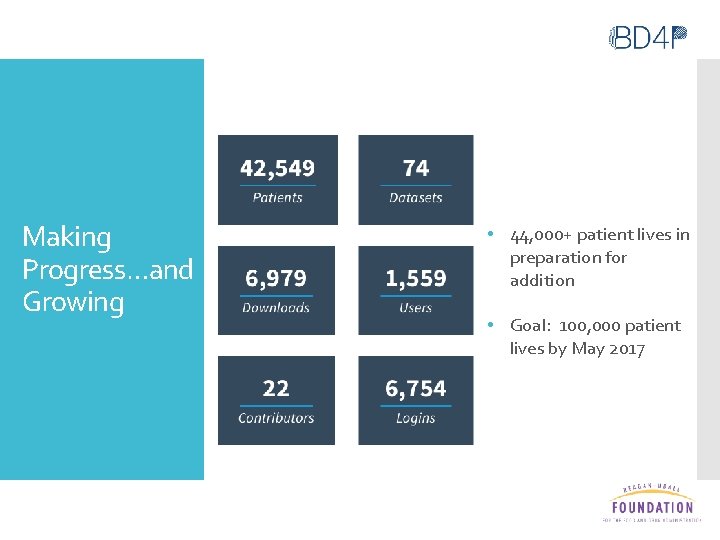
Making Progress…and Growing • 44, 000+ patient lives in preparation for addition • Goal: 100, 000 patient lives by May 2017

SHARE For Cures “The estimated 40, 000 women (and a few men) who die annually [from breast cancer] can’t wait years for FDA-approved, ‘gold standard’ clinical trials. We’re dying now. ” Laurie Becklund As I Lay Dying Los Angeles Times Op-Ed February 20, 2015

There is a growing movement in all aspects of healthcare around using the “siloed” data that is captured to benefit everyone – especially in the patient community SHARE For Cures Above all patients want and need more progress to be made in research We see this movement in the press every day At the same time…we live in an era of growing data and public privacy concerns There are good reasons for those concerns – data is being stolen and in most causes legally bought and sold without their consent or knowledge

Existing issues that SHARE For Cures is trying to address: Researchers Expensive and difficult to gather and analyze large datasets Barriers to Progress Lack of standards/interoperability Expensive licensing fees and/or technology costs Patients Lack of transparency and engagement in current models Limited opportunities to participate in the research process Advocacy Organizations Want opportunities to advance research for their communities in innovative ways – but often lack training or funding

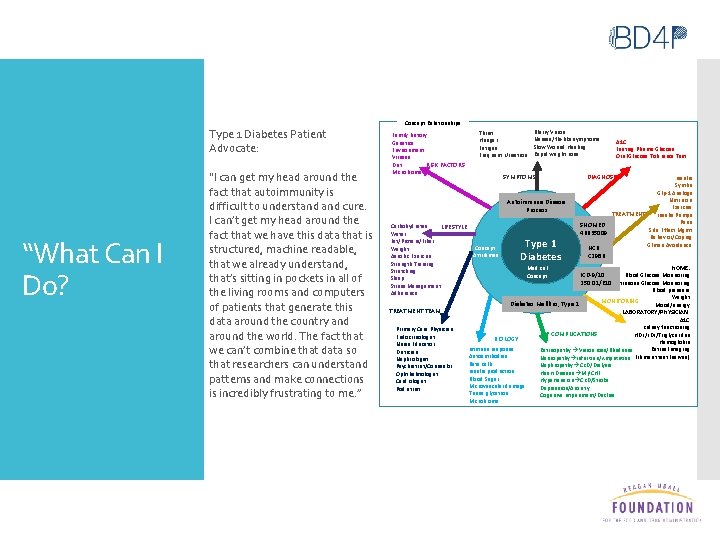
Type 1 Diabetes Patient Advocate: “What Can I Do? “I can get my head around the fact that autoimmunity is difficult to understand cure. I can’t get my head around the fact that we have this data that is structured, machine readable, that we already understand, that’s sitting in pockets in all of the living rooms and computers of patients that generate this data around the country and around the world. The fact that we can’t combine that data so that researchers can understand patterns and make connections is incredibly frustrating to me. ” Concept Relationships Family history Genetics Environment Viruses RISK FACTORS Diet Microbiome Thirst Hunger Fatigue Frequent Urination Blurry Vision Nausea/flu-like symptoms Slow Wound Healing Rapid weight Loss SYMPTOMS Autoimmune Disease Process Carbohydrates LIFESTYLE Water Fat/Protein/Fiber Concept Weight Attributes Aerobic Exercise Strength Training Stretching Sleep Stress Management Adherence TREATMENT TEAM Primary Care Physician Endocrinologist Nurse Educator Dietician Nephrologist Psychiatrist/Counselor Ophthalmologist Cardiologist Podiatrist Type 11 Type Diabetes Medical Concept A 1 C Fasting Plasma Glucose Oral Glucose Tolerance Test DIAGNOSIS Insulin Symlin Glp-1 Analogs Nutrition Exercise TREATMENT Insulin Pumps Pens SNOMED Side Effect Mgmt 46635009 Behavior/Coping Gluten Avoidance NCIt C 2986 HOME: Blood Glucose Monitoring ICD-9/10 250. 01/E 10 Continuous Glucose Monitoring Blood pressure Weight Diabetes Mellitus, Type 1 Mood/energy LABORATORY/PHYSICIAN: A 1 C Kidney functioning COMPLICATIONS HDL/LDL/Triglycerides BIOLOGY Hemoglobin Immune response Retinal Imaging Retinopathy Vision Loss/Blindness Autoantibodies Neuropathy Infection/Amputation Filament test (nerves) Beta cells Nephropathy CKD/Dialysis Insulin production Heart Disease MI/CHF Blood Sugar Hypertension CKD/Stroke Microvascular damage Depression/Anxiety Tissue glycation Cognitive Impairment/Decline Microbiome MONITORING

68% of 3, 000 respondents – “Over twothirds…would be willing to share their health information anonymously with researchers. ” Source: Truven Data Privacy Health Poll 11/2014 The Right Time 81% of chronically ill patients and 67% of those not chronically ill want the ability to both extract and annotate their clinical records for their own use. Source: Accenture Patient Engagement Survey 2015 90% of researchers state there is value in having access to clinical, pharmaceutical, wellness, and lifestyle data…but the barriers are too great. Source: Harvard Business Review 2016

SHARE For Cures Mission SHARE For Cures To empower you to use your health data to advance medical research and save lives. SHARE For Cures Transformational Vision Crowdsourcing cures by empowering everyday people to drive medical research forward.

To accomplish all we have set out to do, we knew we needed to create… SHARE For Cures A way for you to access your health data all in one place and make a safe, secure, hassle-free, cost-free, and meaningful contribution to disease research now SHARE – System for Health And Research data Exchange A simple UI and a powerful platform to engage patients and aggregate real-world data from many sources and enable patientdriven data sharing for research

Consent SHARE For Cures uses a general consent so that participants can opt-in to sharing their data With researchers – participants can revoke sharing consent at any time Sharing Preferences – participants can identify how/where they will allow their data to be shared

Participants can upload their data (including EMRs) directly from their medical providers Connects to ~50% of hospitals in the US Also CVS, Walgreens, Rite. Aid, Quest labs, etc. Adding Medical Data Participants can also upload data from health apps Fitbit, Runkeeper, 23 and. Me, Google Fit, Apple Watch, etc. This information will display in the participant’s SHARE account They can determine who has access to their data, and for what purposes (i. e. all uses, research, with identifiable data)

Other Current User. Facing Features Ability to view and download your data from all connected sources Ability to annotate or add to your data Graphical views of your data Surveys “Facebook Style” Feed – future use for educational materials, clinical trial opportunities, etc.

SHARE Button to seamlessly connect with other patient-facing platforms Caregiver/Proxy logins Future Plans Mobile App Researcher Portal Enhanced Artificial-Intelligence Algorithms Citizen Scientist Portal

Users have complete control over who can use their data, and visibility into who has seen it and why. Legal & Ethical Considerations General legal consent (direct patient authorization, outside of HIPAA) for data sharing based on preferences. Specific studies request datasets from SFC but get their own IRB approval/waiver. Built-in ability to gather up-front consent for specific studies using the study consent documents. Currently, all agreements are for research purposes. More legal work to be done if data is used for clinical care.

Infrastructure in Amazon’s HIPAA-compliant cloud Built with HITRUST controls in mind. Privacy & Security HITRUST encompasses HIPAA, HITECH, Meaningful Use, Individual State Laws, PCI, ISO, and COBIT Pursuing HITRUST certification Users have complete control over who can use their data, and visibility into who has seen it and why.

Other Patient -Directed Data Sharing Disease-Specific Projects & Registries Personal Health Records

Project Data Sphere Discussion: Compare & Contrast SHARE For Cures Think about: • Mechanisms of Consent • Privacy • Data Collected (remember the Vs) • Data Sharing • Patient Data Access