Patient Eligibility for Commercial TAVR in the US

Patient Eligibility for Commercial TAVR in the US Michael Mack, M. D. Baylor Healthcare System Dallas, TX February 25, 2013

Michael J. Mack, MD I/we have no real or apparent conflicts of interest to report.

TAVR Approved in the U. S “The FDA Label” • November 2, 2011 – Edwards SAPIEN valve – Transfemoral delivery – Patients with severe symptomatic native aortic valve stenosis – Determined by a cardiac surgeon to be inoperable for open aortic valve replacement – Existing co-morbidities would not preclude the expected benefit from correction of the aortic stenosis

TAVR Approved in the U. S “The FDA Label” October 19, 2012 – Edwards SAPIEN valve – Transfemoral delivery – Transapical delivery – Patients with severe symptomatic native aortic valve stenosis – Determined by a cardiac surgeon to high risk for operation for open aortic valve replacement – Existing co-morbidities would not preclude the expected benefit from correction of the aortic stenosis

• • • TAVR approved under “coverage with evidence development” Approved for treatment of severe symptomatic aortic stenosis FDA approved indication and with an FDA approved device Two cardiac surgeons approve Performed in facility with • >50 surgical AVR’/year (~400 centers) • >400 caths/50 PCI/year • >20 TAVR/year • Mortality <15% • Stroke <15% • Multidisciplinary Heart Team • Mandatory National TVT Registry participation

Who Is Eligible for Commercial TAVR in U. S. ? • Patients with severe, symptomatic, native aortic valve stenosis who are declared by a cardiac surgeon to be: • Inoperable– Can be treated by a TF approach • High Risk Operable – Can be treated by a TF or TA Approach

Who is NOT Eligible? • • • Asymptomatic patients Non-native valve disease Patients not high risk or inoperable Inoperable patients for non-TF approaches High risk patients for access other than TF or TA

Who Is Covered for Reimbursement by CMS? • FDA approved indication and with an FDA approved device • Two cardiac surgeons approve • Centers meeting credentialing criteria and outcomes • Heart Team • Participation in TVT Registry

Who Is NOT Covered? • CMS NCD criteria not met • Those patients not included in the “FDA label”i. e. , “off-label” use

How Does Coverage Expand ? • “Coverage with evidence development” • Expand the FDA labeled indication • IDE studies to expand the label

STS/ACC TVT Registry Sponsored IDE Studies • FDA Approved – Alternative Access to TF in Inoperable Patients • In Process of Submission – TAVR in Degenerated Surgical Aortic Valve Prosthesis – Alternative Access other than TF and TA in High Risk Operable Patients
TVT National Registry • Comprehensive prospective observational database (7 -page CRF) • FU includes 30 -days, 1 -year (incl. QOL measures) • TVT compliance linked to reimbursement

David Holmes President American College of Cardiology 2011 Jeff Shuren Director CDRH FDA Michael Mack President Society of Thoracic Surgeons 2011

• UDI system incorporated into EHR • National and international device registries • Modernize adverse event reporting • New methods for evidence generation, synthesis and appraisal
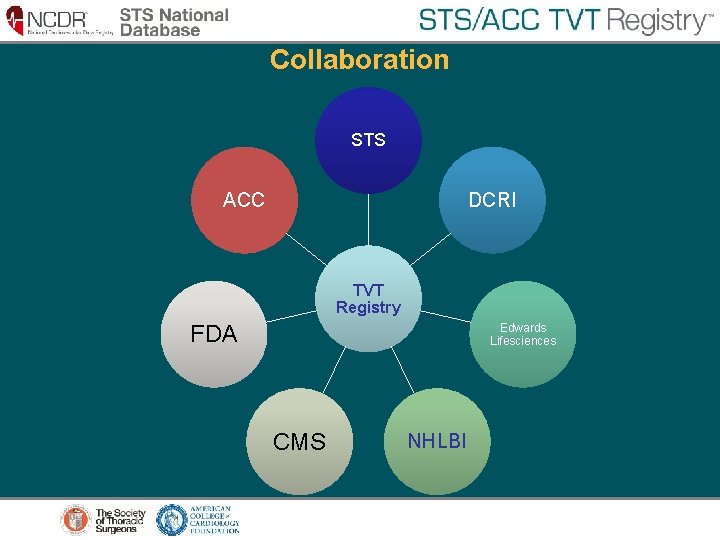
Collaboration STS ACC DCRI TVT Registry FDA Edwards Lifesciences CMS NHLBI

Steering Committee STS Members ACC Members • • Fred Edwards Fred Grover Michael Mack Dave Shahian Ralph Brindis John Carroll David Holmes Murat Tuzcu Ex Officio Members • • Registry Operations Center FDA - Danica Marinac-Dabic CMS - Jyme Schafer NHLBI - Frank Evans Chair TVT R&P Subcommittee - John Rumsfeld • DCRI – Eric Peterson, Matt Brennan • NCDR Data Analytics Center • DCRI Staff • • Cynthia Shewan, Hilary Kirk Joan Michaels, Kathleen Hewitt, Barb Christensen
Current Status February, 2013 • 178 U. S. TAVR sites enrolled sites • 3, 116 patient records • Nested registry for PAS 2 study for Edwards Sapien Valve
Next Steps • Annual reports at society annual meetings • Linkage with CMS database for long term outcomes • Develop TAVR risk model • Global harmonization of this registry with OUS databases/studies based on VARC common definitions- April 22, 2013 • Extension to pre-market use • Link with STS Adult Cardiac database for comparative effectiveness with surgical AVR
- Slides: 18