Pathophysiology of the Lung parenchyma Dr Celia Lanteri






- Slides: 42

Pathophysiology of the Lung parenchyma Dr Celia Lanteri Austin, Alfred & Epworth Hospitals, Victoria, Australia CRFS Physiology Review Workshop, Melbourne 2008 Australian & New Zealand Society of Respiratory Science

Parenchymal disease Q 1. Pathophysiology of the lung can be related to: A. Problems with air getting in B. Problems with air getting out C. Problems with gas/blood going round and round D. All of the above • Obstructive & restrictive lung disease ANZSRS, Melbourne 2008

Pathology patterns • Obstructive lung disease – Usually referring to primary airway problem – Eg. Asthma, chronic bronchitis – But includes emphysema • Restrictive lung disease – Parenchymal pathology – Chest wall problem – Muscle weakness ANZSRS, Melbourne 2008

Other causes of parenchymal pathology • Infective lung disease • Pulmonary vascular disease • Connective tissue diseases • Inflammatory disease • Malignancy ANZSRS, Melbourne 2008

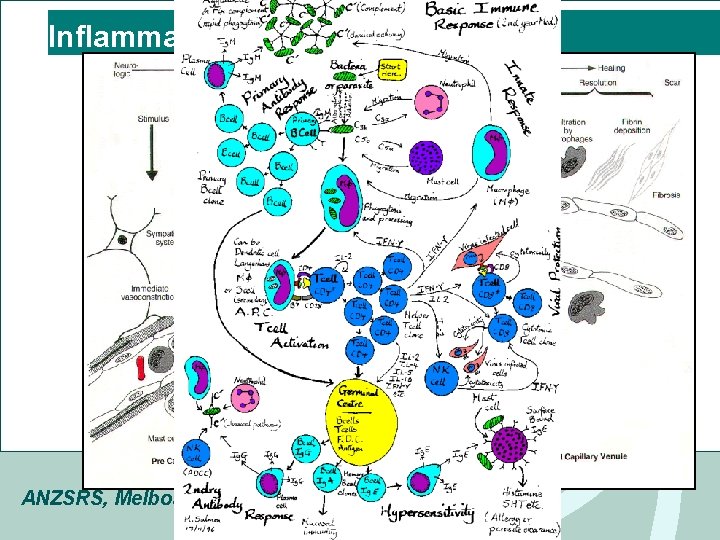
Inflammation – the basics • Injury: attraction of acute inflammatory cells - Neutrophils, macrophages • Vasodilatation & oedema – Capillary leak • Recruitment of other inflammatory mediator cells and regulatory cells • Migration of fibroblasts cells and chronic inflammatory reaction • Organisation, repair or scarring ANZSRS, Melbourne 2008

Inflammation ANZSRS, Melbourne 2008

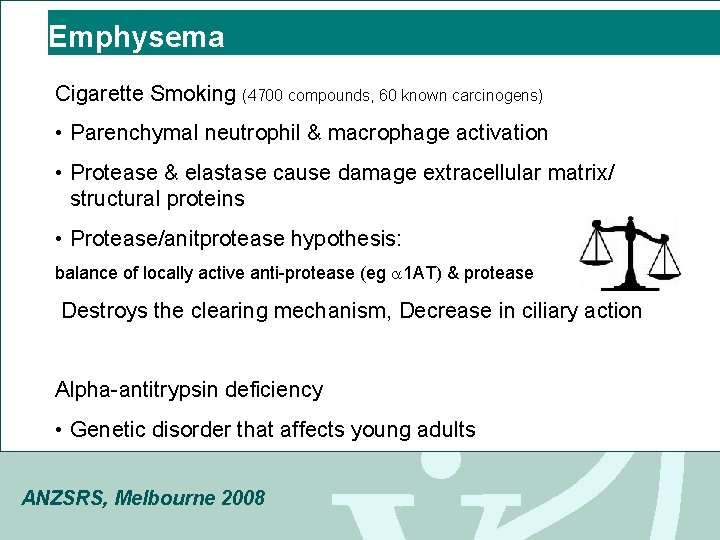
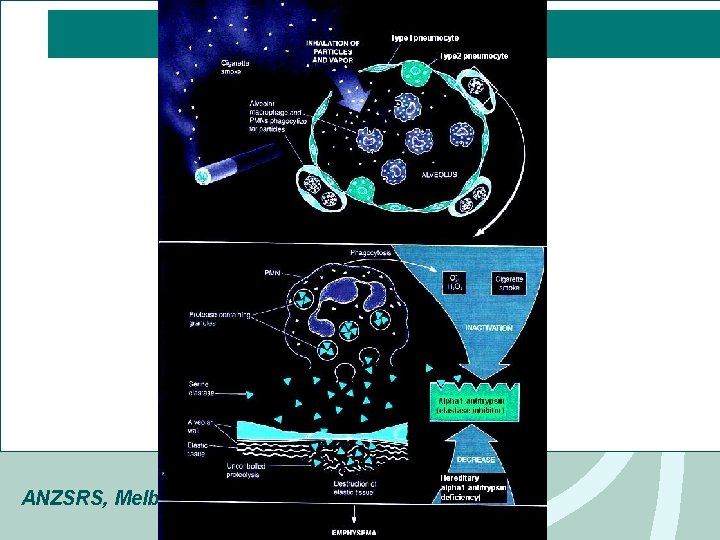
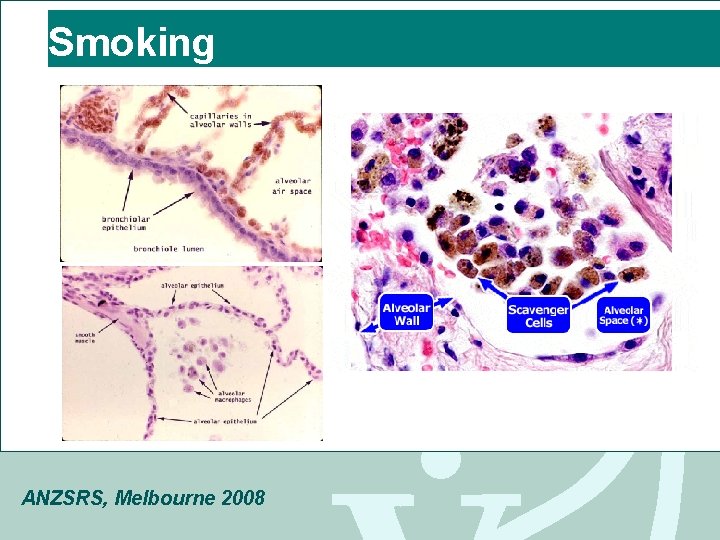
Emphysema Cigarette Smoking (4700 compounds, 60 known carcinogens) • Parenchymal neutrophil & macrophage activation • Protease & elastase cause damage extracellular matrix/ structural proteins • Protease/anitprotease hypothesis: balance of locally active anti-protease (eg 1 AT) & protease Destroys the clearing mechanism, Decrease in ciliary action Alpha-antitrypsin deficiency • Genetic disorder that affects young adults ANZSRS, Melbourne 2008

ANZSRS, Melbourne 2008

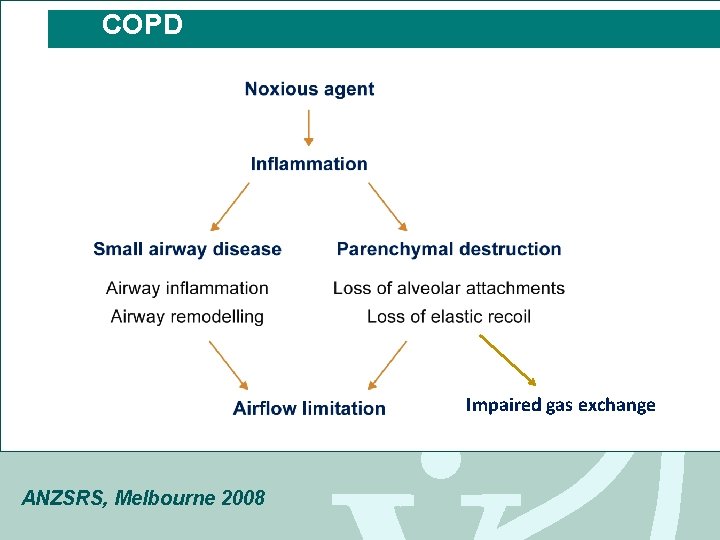
COPD Impaired gas exchange ANZSRS, Melbourne 2008

Smoking ANZSRS, Melbourne 2008

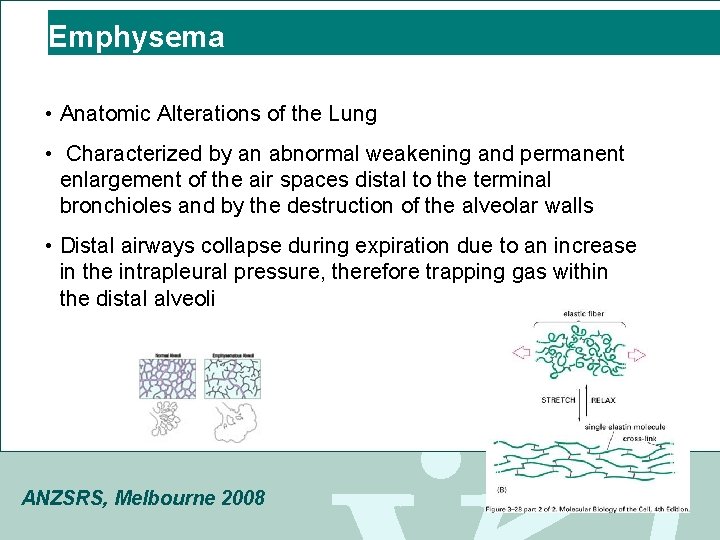
Emphysema • Anatomic Alterations of the Lung • Characterized by an abnormal weakening and permanent enlargement of the air spaces distal to the terminal bronchioles and by the destruction of the alveolar walls • Distal airways collapse during expiration due to an increase in the intrapleural pressure, therefore trapping gas within the distal alveoli ANZSRS, Melbourne 2008

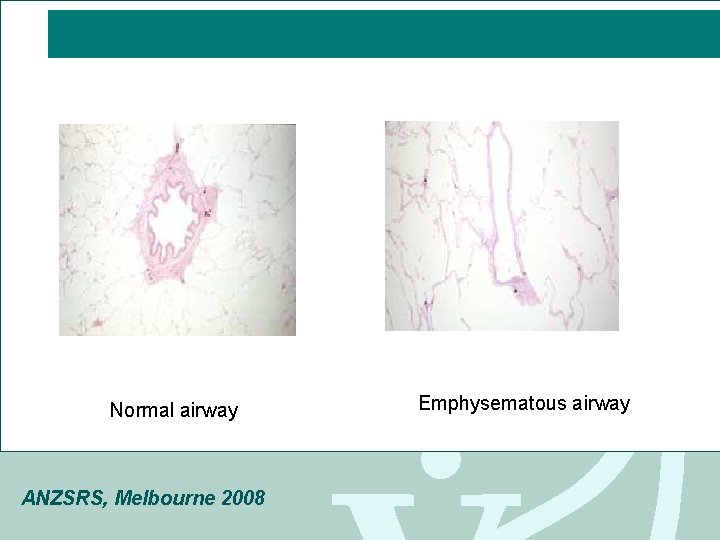
Normal airway ANZSRS, Melbourne 2008 Emphysematous airway

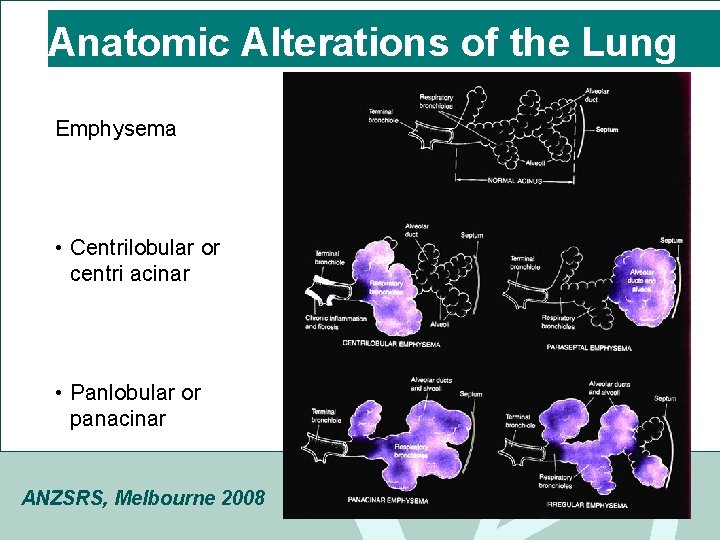
Anatomic Alterations of the Lung Emphysema • Centrilobular or centri acinar • Panlobular or panacinar ANZSRS, Melbourne 2008

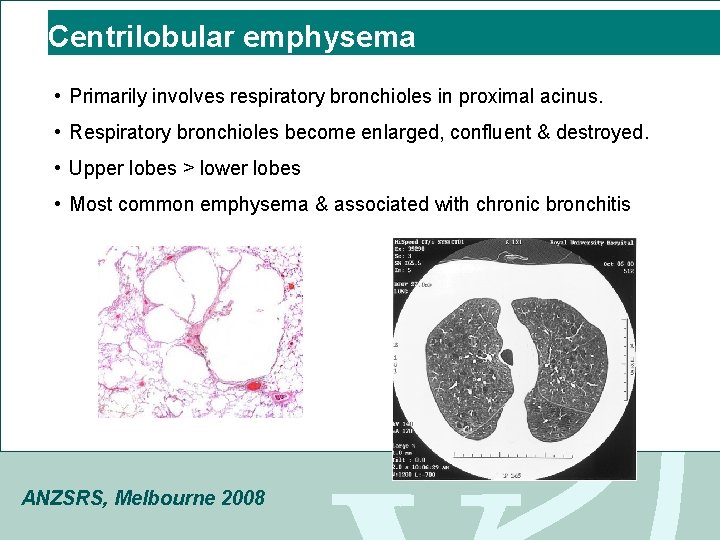
Centrilobular emphysema • Primarily involves respiratory bronchioles in proximal acinus. • Respiratory bronchioles become enlarged, confluent & destroyed. • Upper lobes > lower lobes • Most common emphysema & associated with chronic bronchitis ANZSRS, Melbourne 2008

Panlobular emphysema MARIJUANA LUNG • weakening and enlargement of all of the air spaces distal to the terminal bronchioles MARIJUANA LUNG • Usually found in the lower parts of the lung • May be associated with alpha 1 antitrypsin deficiency • Bullous emphysema (>1 cm) ANZSRS, Melbourne 2008

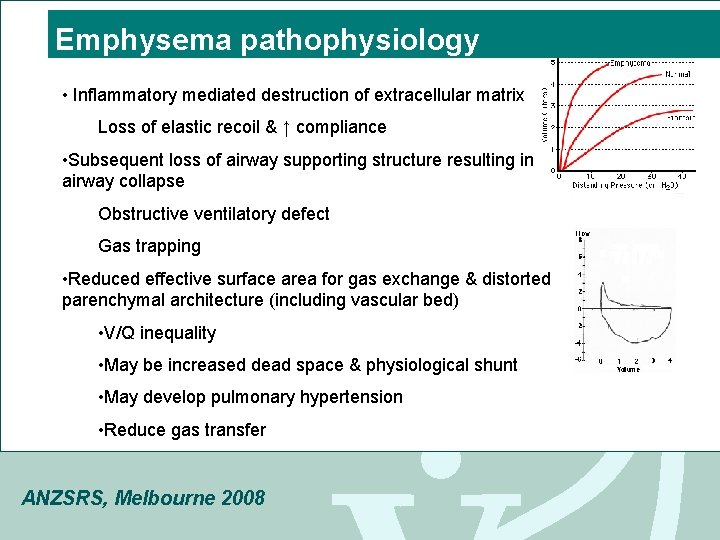
Emphysema pathophysiology • Inflammatory mediated destruction of extracellular matrix Loss of elastic recoil & ↑ compliance • Subsequent loss of airway supporting structure resulting in airway collapse Obstructive ventilatory defect Gas trapping • Reduced effective surface area for gas exchange & distorted parenchymal architecture (including vascular bed) • V/Q inequality • May be increased dead space & physiological shunt • May develop pulmonary hypertension • Reduce gas transfer ANZSRS, Melbourne 2008

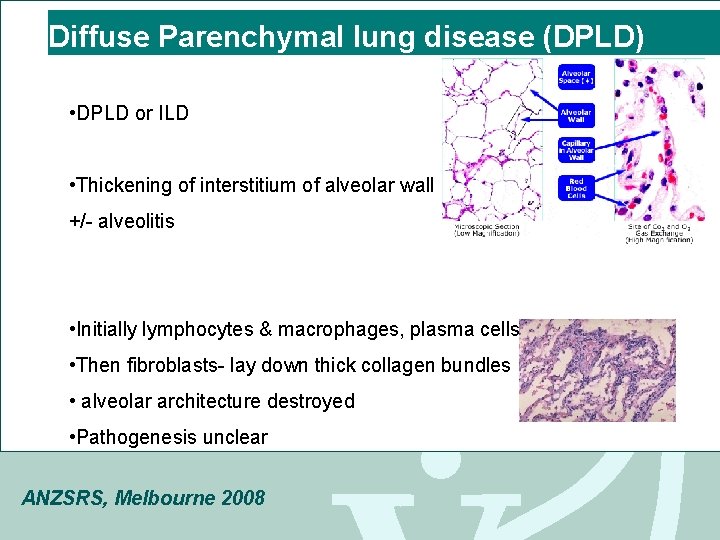
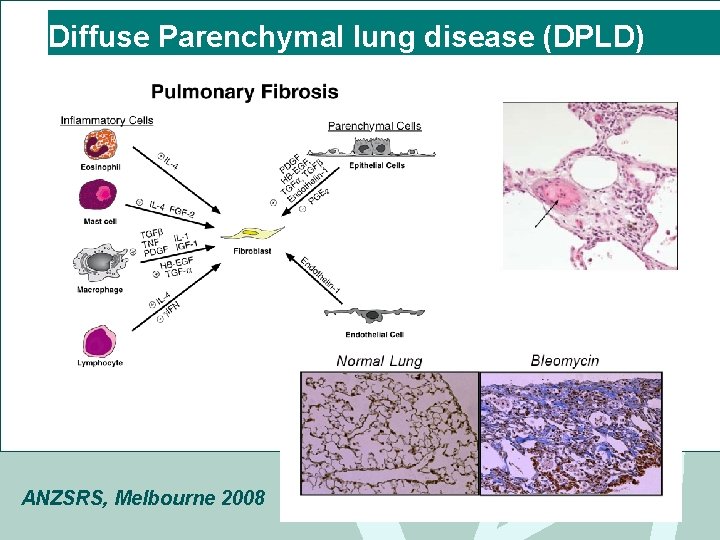
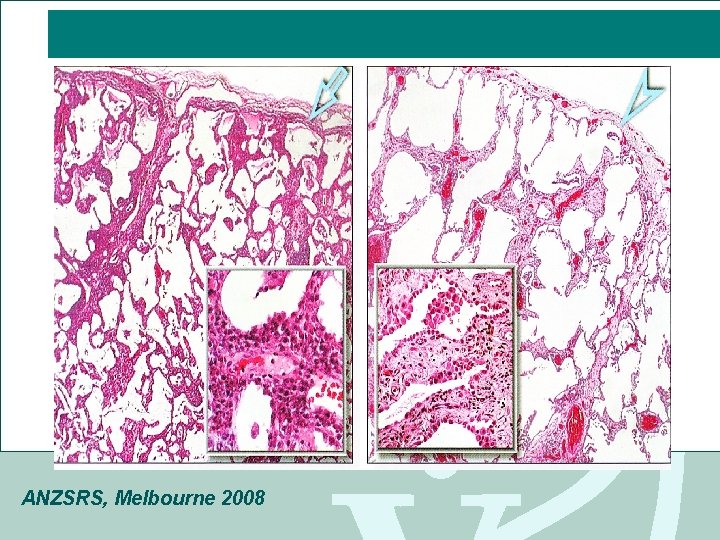
Diffuse Parenchymal lung disease (DPLD) • DPLD or ILD • Thickening of interstitium of alveolar wall +/- alveolitis • Initially lymphocytes & macrophages, plasma cells • Then fibroblasts- lay down thick collagen bundles • alveolar architecture destroyed • Pathogenesis unclear ANZSRS, Melbourne 2008

Diffuse Parenchymal lung disease (DPLD) ANZSRS, Melbourne 2008

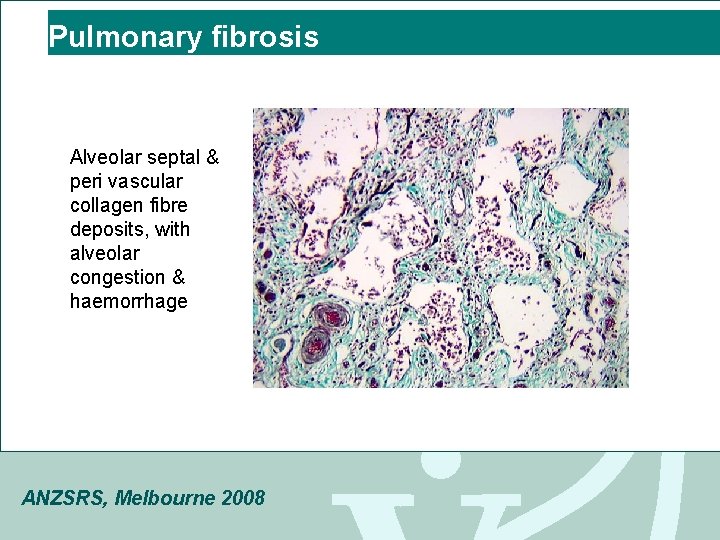
Pulmonary fibrosis Alveolar septal & peri vascular collagen fibre deposits, with alveolar congestion & haemorrhage ANZSRS, Melbourne 2008

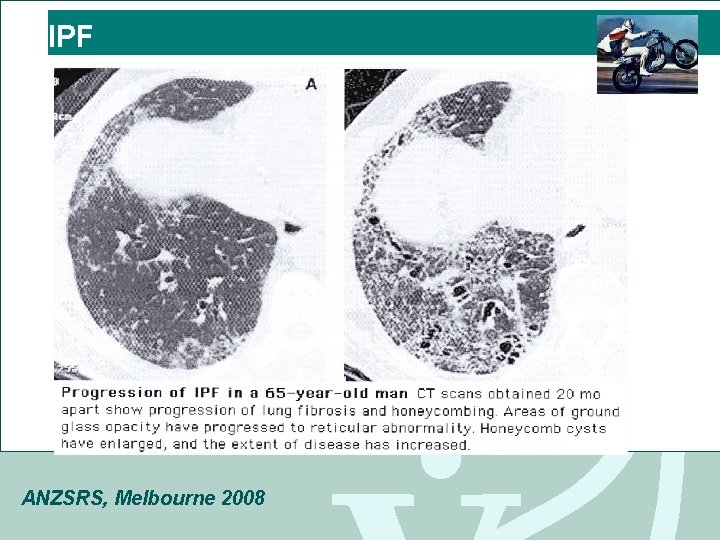
IPF ANZSRS, Melbourne 2008

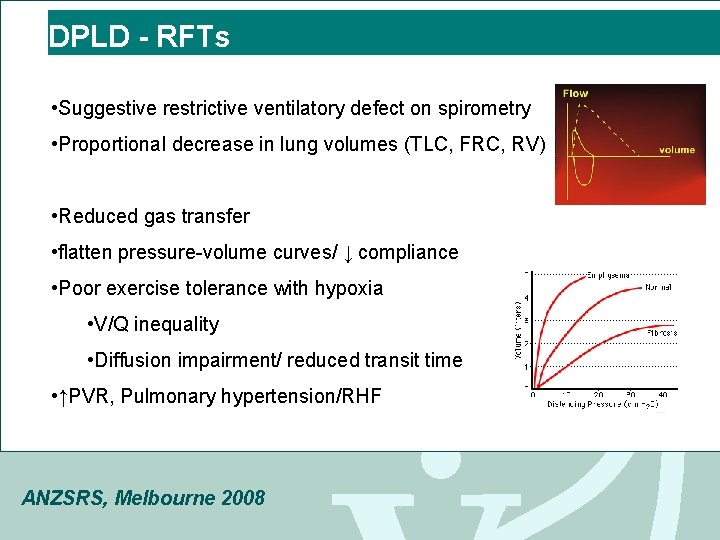
DPLD - RFTs • Suggestive restrictive ventilatory defect on spirometry • Proportional decrease in lung volumes (TLC, FRC, RV) • Reduced gas transfer • flatten pressure-volume curves/ ↓ compliance • Poor exercise tolerance with hypoxia • V/Q inequality • Diffusion impairment/ reduced transit time • ↑PVR, Pulmonary hypertension/RHF ANZSRS, Melbourne 2008

Diffuse Parenchymal lung disease (DPLD) • DPLD or ILD • Known cause • Toxins – radiation, drugs: MXT, bleomycin, amiodarone, nitrofurantoin • Occupational (pneumoconiosis): asbestos, silica, coal workers lung • Connective tissue disease: eg. RA, SLE, scleroderma • Inflammatory: eg. Vasculitis, sarcoid • Hypersensitivity pneumonitis / EAA • Idiopathic Interstitial pneumonia • IPF/CFA(=UIP), NSIP, RBILD, DIP, AIP, LIP ANZSRS, Melbourne 2008

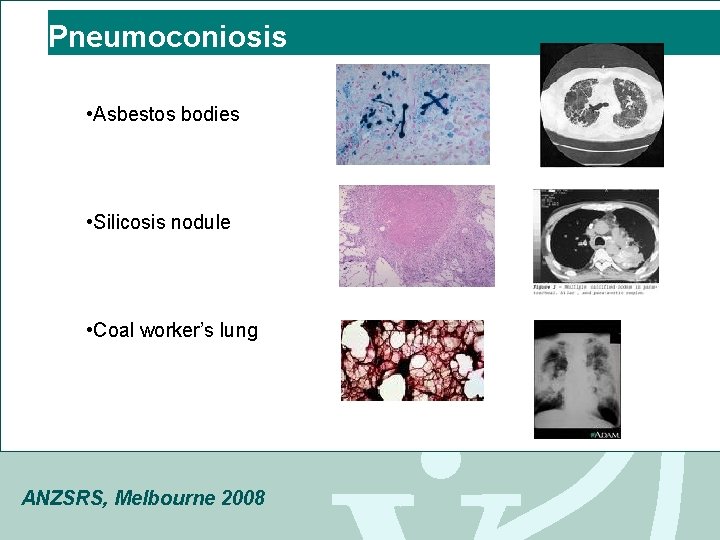
Pneumoconiosis • Asbestos bodies • Silicosis nodule • Coal worker’s lung ANZSRS, Melbourne 2008

Scleroderma / CTD • Limited Sc: 10% develop PAH late in course of disease (independent of ILD) Mortality rate ~60% at five years • Diffuse Sc (SSc): Pulmonary disease in 30 -70% patients ILD & pulmonary vascular disease Surpassed renal involvement as most common cause of death Associated with poorer prognosis • Steroids/cyclophosphamide Rx early – before established fibrosis ANZSRS, Melbourne 2008

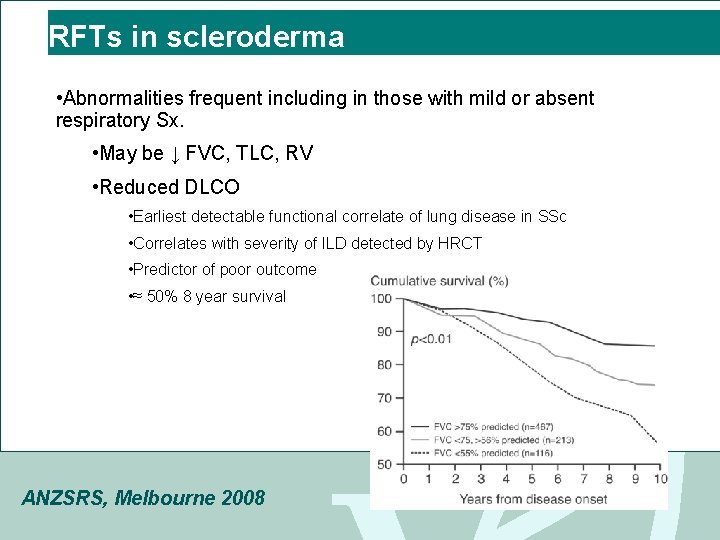
RFTs in scleroderma • Abnormalities frequent including in those with mild or absent respiratory Sx. • May be ↓ FVC, TLC, RV • Reduced DLCO • Earliest detectable functional correlate of lung disease in SSc • Correlates with severity of ILD detected by HRCT • Predictor of poor outcome • ≈ 50% 8 year survival ANZSRS, Melbourne 2008

ANZSRS, Melbourne 2008

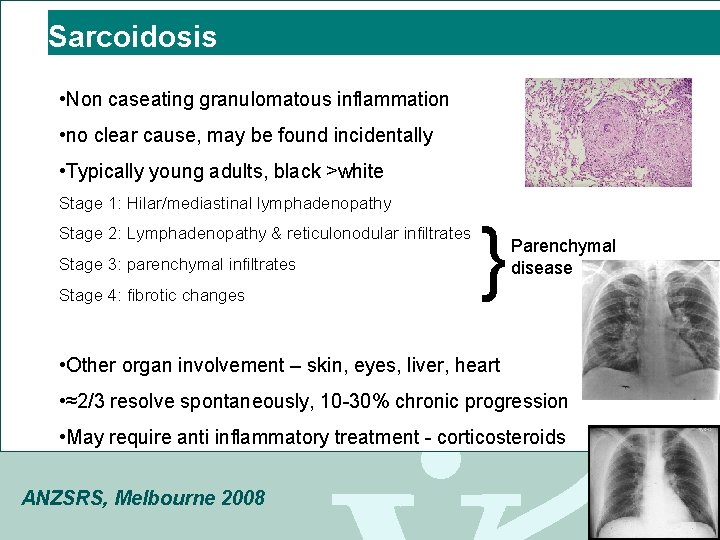
Sarcoidosis • Non caseating granulomatous inflammation • no clear cause, may be found incidentally • Typically young adults, black >white Stage 1: Hilar/mediastinal lymphadenopathy Stage 2: Lymphadenopathy & reticulonodular infiltrates Stage 3: parenchymal infiltrates Stage 4: fibrotic changes } Parenchymal disease • Other organ involvement – skin, eyes, liver, heart • ≈2/3 resolve spontaneously, 10 -30% chronic progression • May require anti inflammatory treatment - corticosteroids ANZSRS, Melbourne 2008

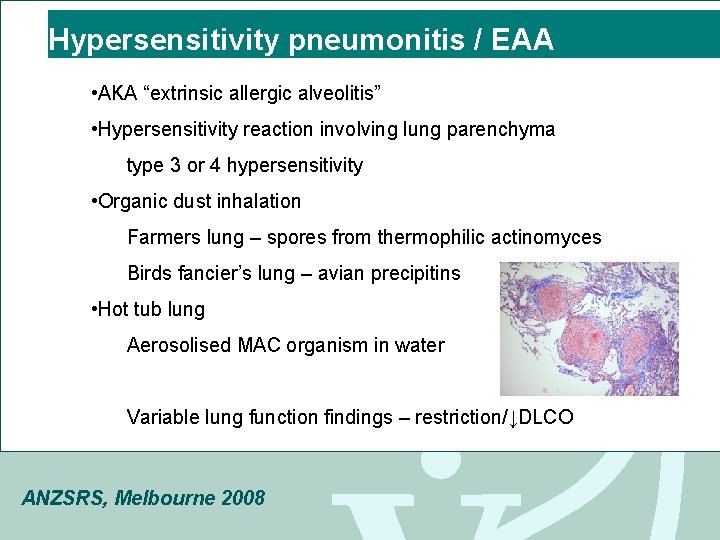
Hypersensitivity pneumonitis / EAA • AKA “extrinsic allergic alveolitis” • Hypersensitivity reaction involving lung parenchyma type 3 or 4 hypersensitivity • Organic dust inhalation Farmers lung – spores from thermophilic actinomyces Birds fancier’s lung – avian precipitins • Hot tub lung Aerosolised MAC organism in water Variable lung function findings – restriction/↓DLCO ANZSRS, Melbourne 2008

Pulmonary vascular disorders • Acute PE Chronic thromboembolic disease Pulmonary hypertension arterial venous ANZSRS, Melbourne 2008

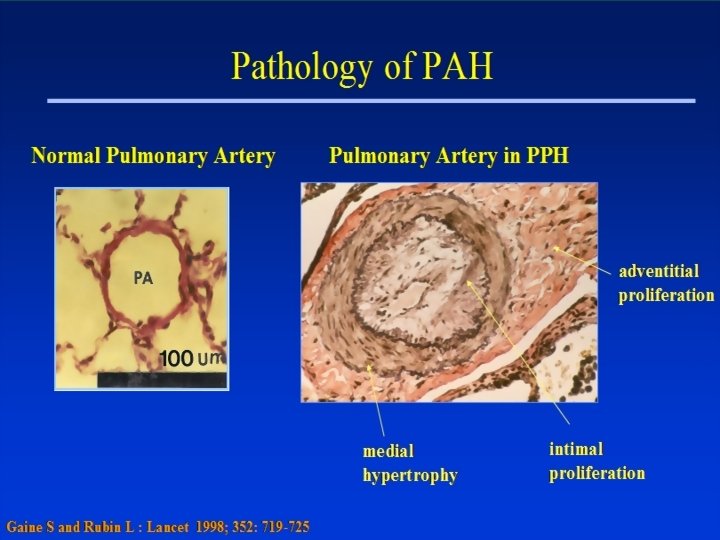
Pulmonary hypertension • Pulmonary arterial hypertension (PAH) – Primary or secondary • Pulmonary venous hypertension (PVH) – LV disease, VOC • PHT associated with diseases of the respiratory system, hypoxia, OSA • Chronic thromboembolic disease • Pulmonary vascular disorders • Miscellaneous! ANZSRS, Melbourne 2008

PAH • Primary PAH (idiopathic) – sporadic – Familial: 6 -10% of PPH, AD, BMPR 2 mutation • Secondary – Connective Tissue Disease (50% of scleroderma pts) – Congenital shunts – Portal HT – HIV infection – Toxins: Anorexic drugs, cocaine, rapeseed – Persistent pulmonary HT of the new born ANZSRS, Melbourne 2008

ANZSRS, Melbourne 2008

Pathophysiology of cancer ANZSRS, Melbourne 2008

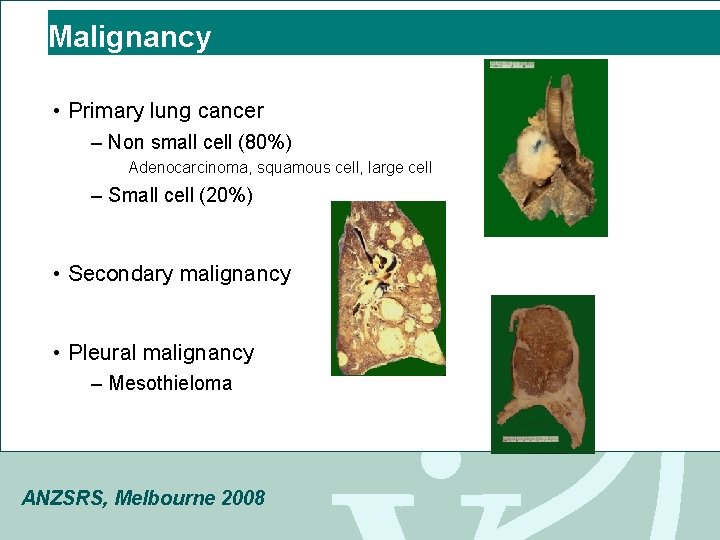
Malignancy • Primary lung cancer – Non small cell (80%) Adenocarcinoma, squamous cell, large cell – Small cell (20%) • Secondary malignancy • Pleural malignancy – Mesothieloma ANZSRS, Melbourne 2008

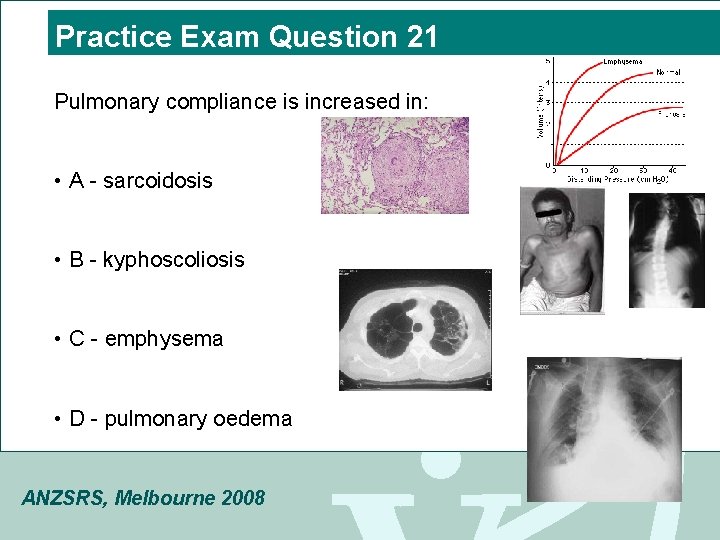
Practice Exam Question 21 Pulmonary compliance is increased in: • A - sarcoidosis • B - kyphoscoliosis • C - emphysema • D - pulmonary oedema ANZSRS, Melbourne 2008

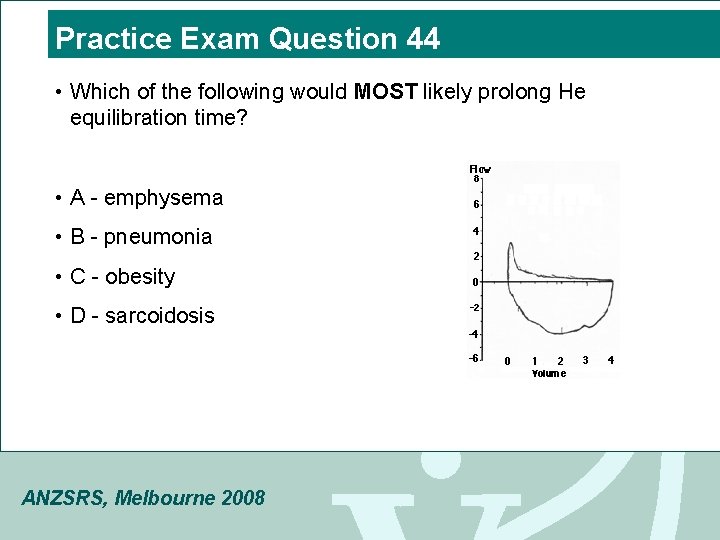
Practice Exam Question 44 • Which of the following would MOST likely prolong He equilibration time? • A - emphysema • B - pneumonia • C - obesity • D - sarcoidosis ANZSRS, Melbourne 2008

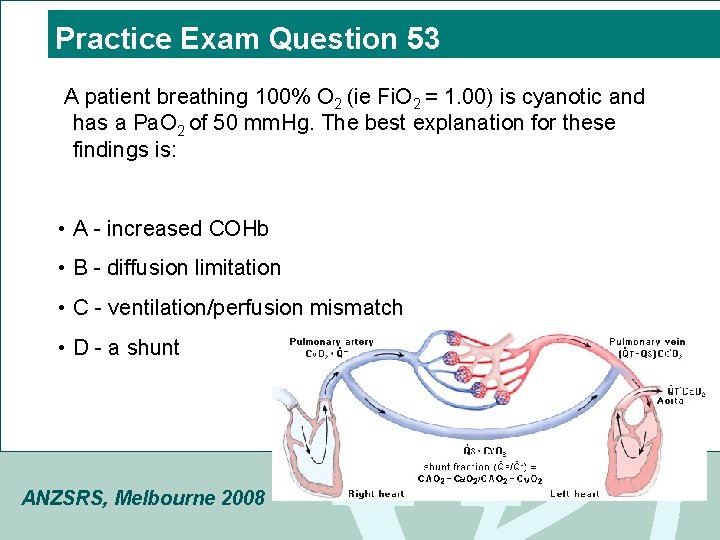
Practice Exam Question 53 A patient breathing 100% O 2 (ie Fi. O 2 = 1. 00) is cyanotic and has a Pa. O 2 of 50 mm. Hg. The best explanation for these findings is: • A - increased COHb • B - diffusion limitation • C - ventilation/perfusion mismatch • D - a shunt ANZSRS, Melbourne 2008

Practice Exam Question 62 DLCO may be decreased in emphysema due to: 1 - increased distance from terminal bronchiole to alveolarcapillary membrane 2 - decreased surface area 3 - loss of pulmonary capillary bed 4 - ventilation/perfusion abnormalities A - 1, 2, 3 and 4 B - 2, 3 and 4 C - 2 and 4 D - 3 only ANZSRS, Melbourne 2008

Practice Exam Question 69 In emphysema there is an increased tendency for the bronchioles to collapse during a forced expiration. This is due to: • A - a decrease in the elastic recoil of the lungs • B - a loss of collagenous tissue from the lungs • C - a failure of bronchiolar chondroblasts • D - excessive tone in the bronchiolar smooth muscle ANZSRS, Melbourne 2008

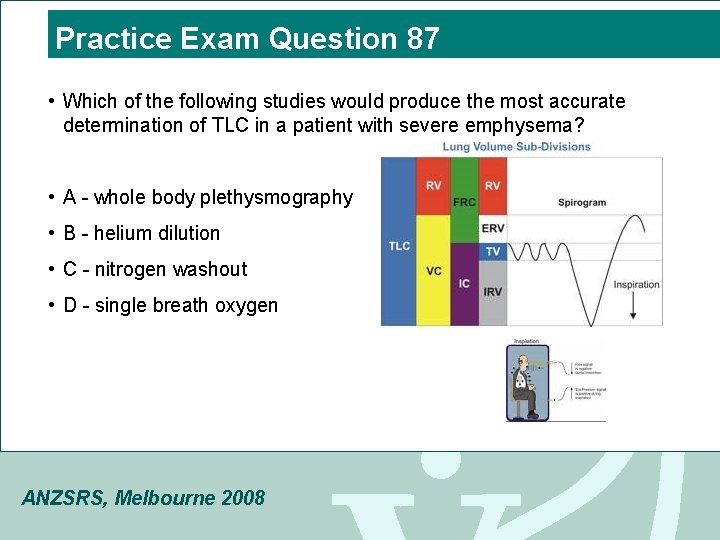
Practice Exam Question 87 • Which of the following studies would produce the most accurate determination of TLC in a patient with severe emphysema? • A - whole body plethysmography • B - helium dilution • C - nitrogen washout • D - single breath oxygen ANZSRS, Melbourne 2008

Practice Exam Question 2 FRC is usually: • A - decreased in patients with airway obstruction • B - higher when measured by plethysmograph than by the helium dilution method in patients with emphysema • C - increased in patients with obesity • D - higher when measured by the helium dilution method than by the nitrogen washout method in patients with airway obstruction ANZSRS, Melbourne 2008

Thanks ANZSRS, Melbourne 2008