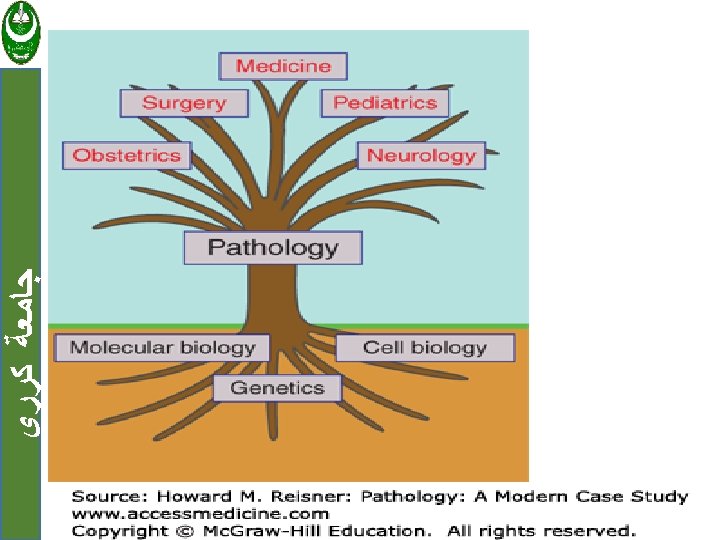
Pathology Definition Scientific study of diseases in term































- Slides: 61


ﺟﺎﻣﻌﺔ ﻛﺮﺭﻱ Pathology Definition : Scientific study of diseases in term of causes and effects


Reference ﺟﺎﻣﻌﺔ ﻛﺮﺭﻱ • Robbins basic pathology 10 edition

ﺟﺎﻣﻌﺔ ﻛﺮﺭﻱ Pathology: Scope General Pathology – Common changes in all tissues. • E. g. . Inflammation, cancer, ageing. Systemic Pathology – Specific changes in organs. • E. g. . Goiter, pneumonia, breast cancer.

ﺟﺎﻣﻌﺔ ﻛﺮﺭﻱ CAUSES OF CELL INJURY: 1. Oxygen Deprivation 2. Chemical Agents 3. Infectious Agents 4. Immunologic Reactions 5. Genetic Defects 6. Nutritional Imbalances 7. Physical Agents 8. Aging Karary University - ( ] ﺍﺳﻢ ﺍﻻﺳﺘﺎﺫ 6

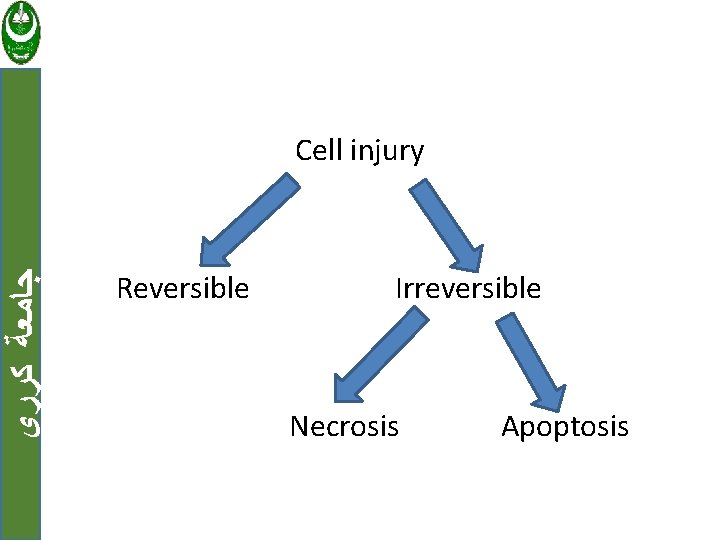
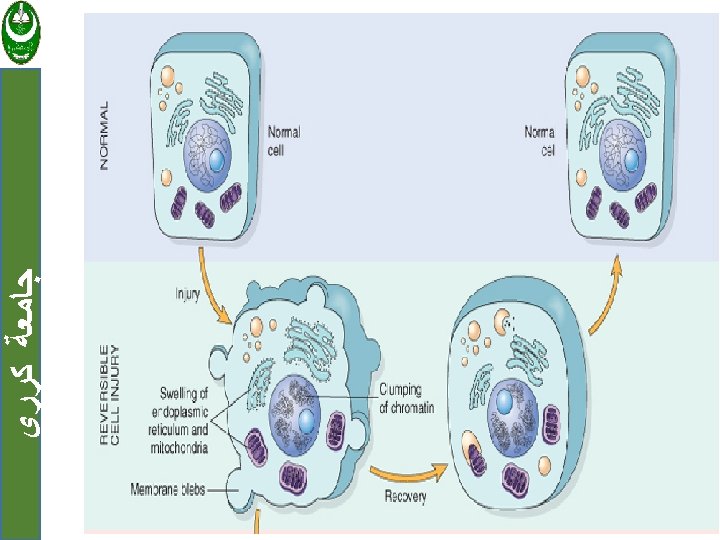
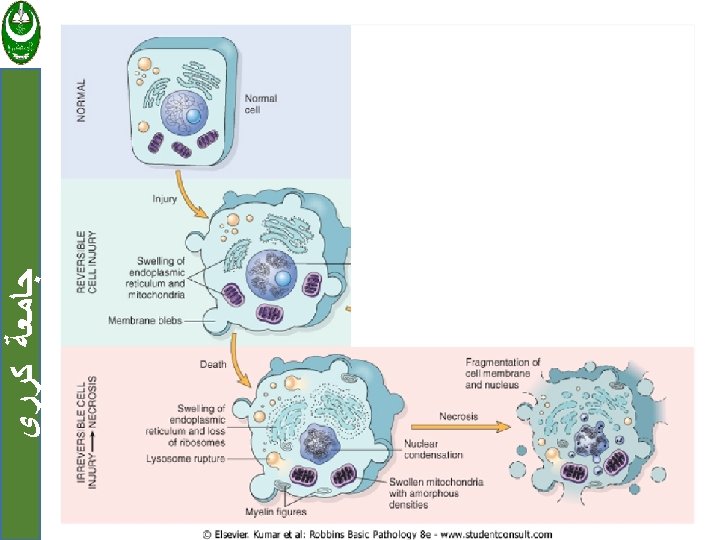
ﺟﺎﻣﻌﺔ ﻛﺮﺭﻱ Cell injury are tow types: 1. Reversible when the injury is limited and cells return to a stable baseline. 2. Irreversible injury; when severe or persistent stress results in death of the affected cells Cell death has tow forms: a. Necrosis b. Apoptosis

ﺟﺎﻣﻌﺔ ﻛﺮﺭﻱ Cell injury Reversible Irreversible Necrosis Apoptosis


ﺟﺎﻣﻌﺔ ﻛﺮﺭﻱ 1. Reversible cell injury Two features of reversible cell injury: a. Cellular swelling. b. Fatty change

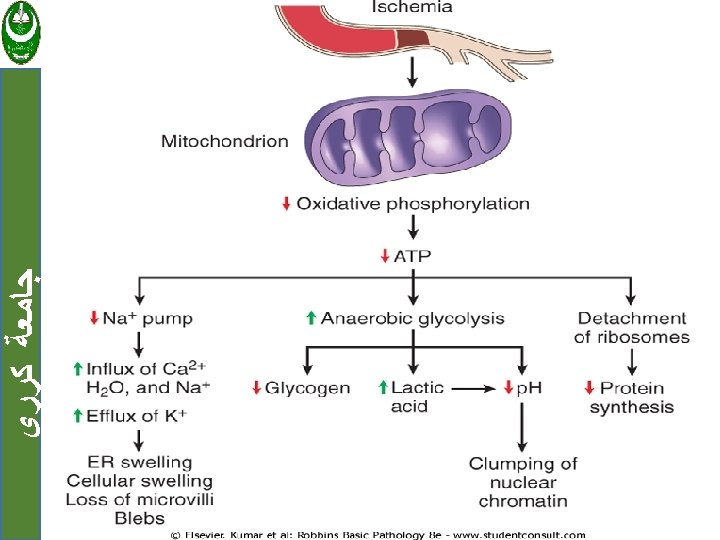
ﺟﺎﻣﻌﺔ ﻛﺮﺭﻱ a. Cellular swelling: Result from failure of energy-dependent ion pumps in the plasma membrane, leading to an inability to maintain ionic and fluid homeostasis.

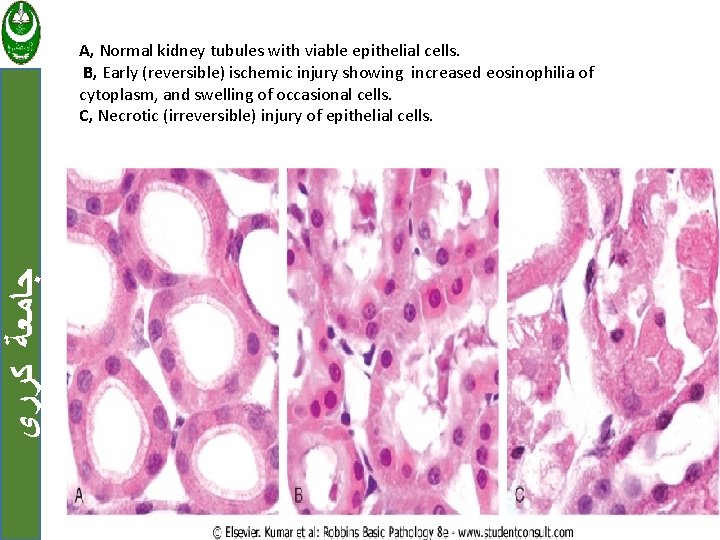
ﺟﺎﻣﻌﺔ ﻛﺮﺭﻱ A, Normal kidney tubules with viable epithelial cells. B, Early (reversible) ischemic injury showing increased eosinophilia of cytoplasm, and swelling of occasional cells. C, Necrotic (irreversible) injury of epithelial cells.

ﺟﺎﻣﻌﺔ ﻛﺮﺭﻱ b. Fatty change: Occurs in hypoxic injury and various forms of toxic or metabolic injury. Manifested by the appearance of small or large lipid vacuoles in the cytoplasm.


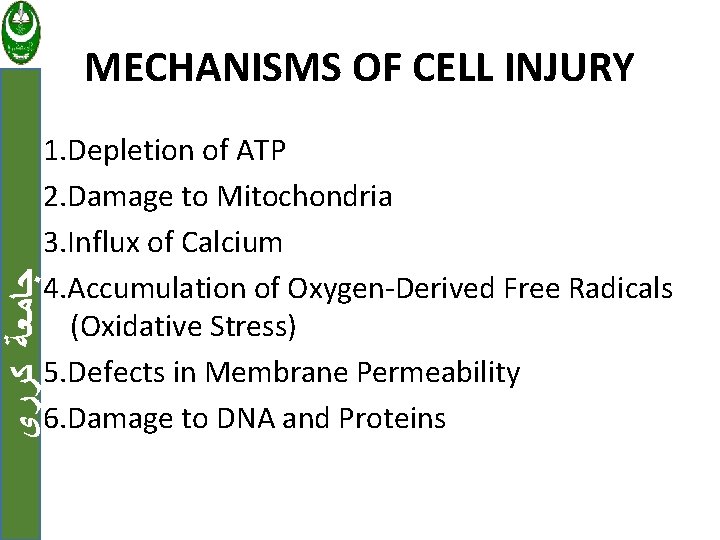
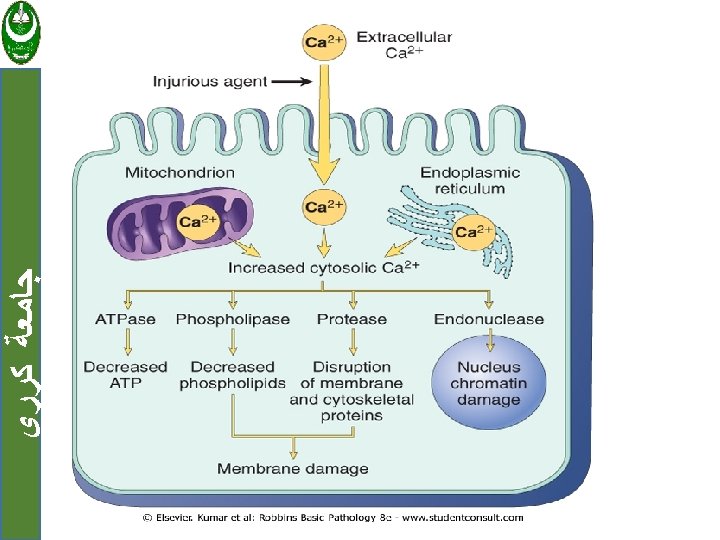
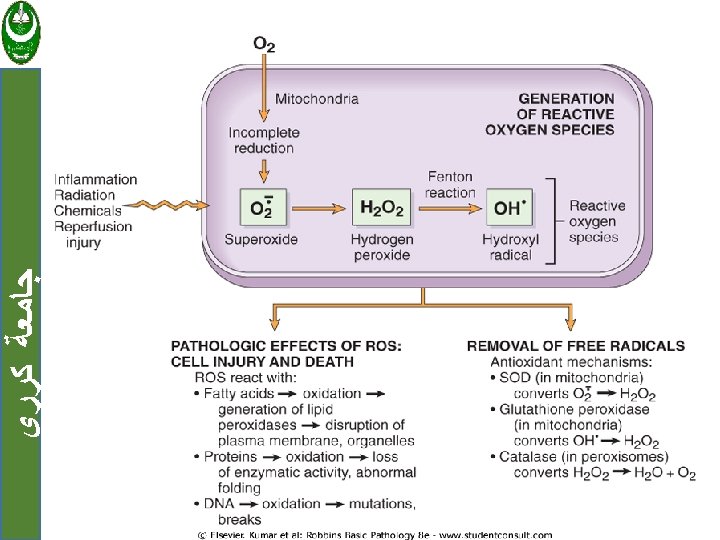
MECHANISMS OF CELL INJURY ﺟﺎﻣﻌﺔ ﻛﺮﺭﻱ 1. Depletion of ATP 2. Damage to Mitochondria 3. Influx of Calcium 4. Accumulation of Oxygen-Derived Free Radicals (Oxidative Stress) 5. Defects in Membrane Permeability 6. Damage to DNA and Proteins




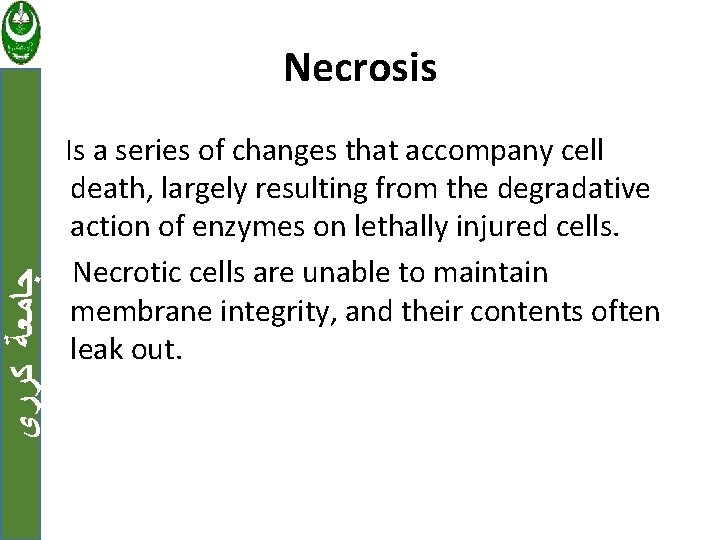
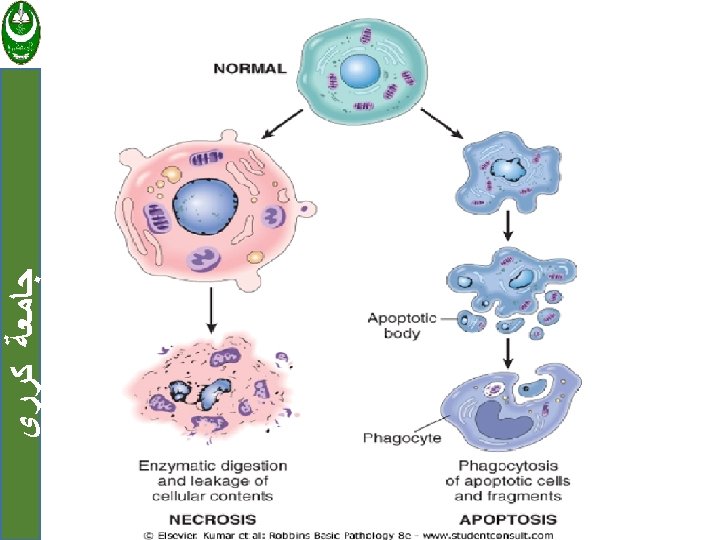
ﺟﺎﻣﻌﺔ ﻛﺮﺭﻱ Necrosis Is a series of changes that accompany cell death, largely resulting from the degradative action of enzymes on lethally injured cells. Necrotic cells are unable to maintain membrane integrity, and their contents often leak out.

ﺟﺎﻣﻌﺔ ﻛﺮﺭﻱ • The enzymes responsible for digestion of the cell are derived either from the lysosomes of the dying cells themselves or from the lysosomes of leukocytes that are recruited as part of the inflammatory reaction to the dead cells.

ﺟﺎﻣﻌﺔ ﻛﺮﺭﻱ • Dead cells may be replaced by large, whorled phospholipid masses, called myelin figures, that are derived from damaged cellular membranes. Also dead cells may calcified

ﺟﺎﻣﻌﺔ ﻛﺮﺭﻱ Nuclear changes assume one of three patterns: 1. karyolysis : fading of chromatin basophilia. 2. pyknosis: characterized by nuclear shrinkage and increased basophilia. 3. karyorrhexis: the nucleus undergoes fragmentation.


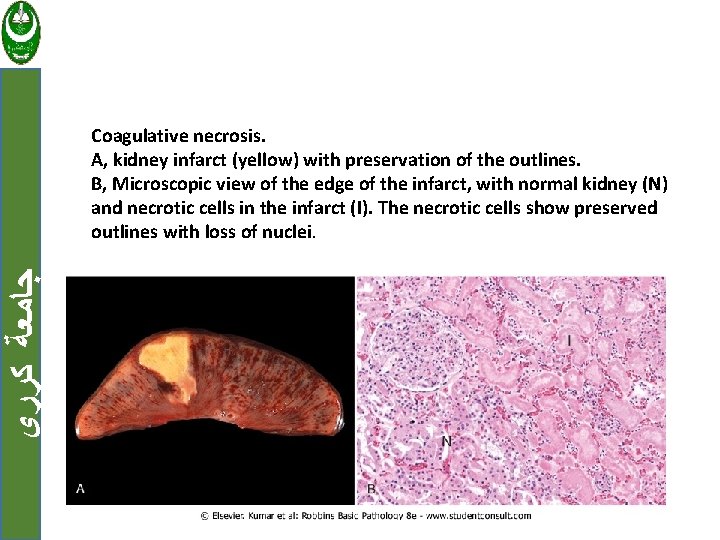
Patterns of Tissue Necrosis: ﺟﺎﻣﻌﺔ ﻛﺮﺭﻱ a. Coagulative necrosis In this form of necrosis the cells are dead but the basic tissue architecture is preserved. Occur in all solid organs except the brain.

ﺟﺎﻣﻌﺔ ﻛﺮﺭﻱ Coagulative necrosis. A, kidney infarct (yellow) with preservation of the outlines. B, Microscopic view of the edge of the infarct, with normal kidney (N) and necrotic cells in the infarct (I). The necrotic cells show preserved outlines with loss of nuclei.

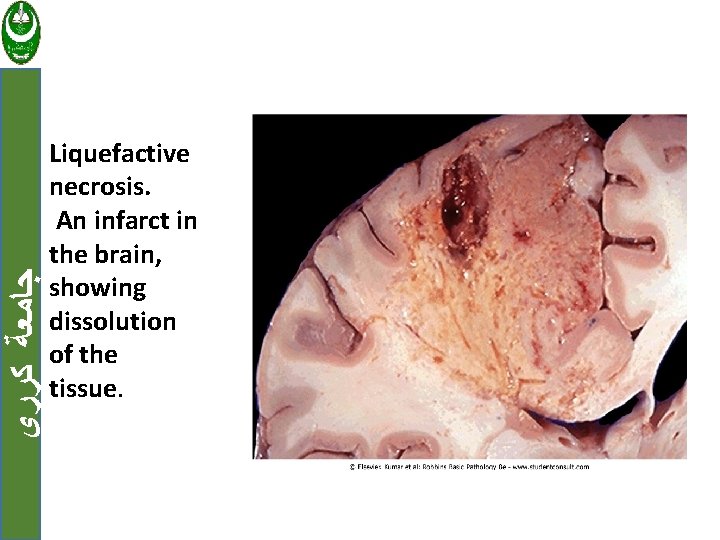
ﺟﺎﻣﻌﺔ ﻛﺮﺭﻱ b. Liquefactive necrosis Is seen in focal bacterial or fungal infections. The microbes stimulate the accumulation of inflammatory cells and the enzymes of leukocytes digest ("liquefy") the tissue. This collection of liquefied material is called pus.

ﺟﺎﻣﻌﺔ ﻛﺮﺭﻱ Liquefactive necrosis. An infarct in the brain, showing dissolution of the tissue.

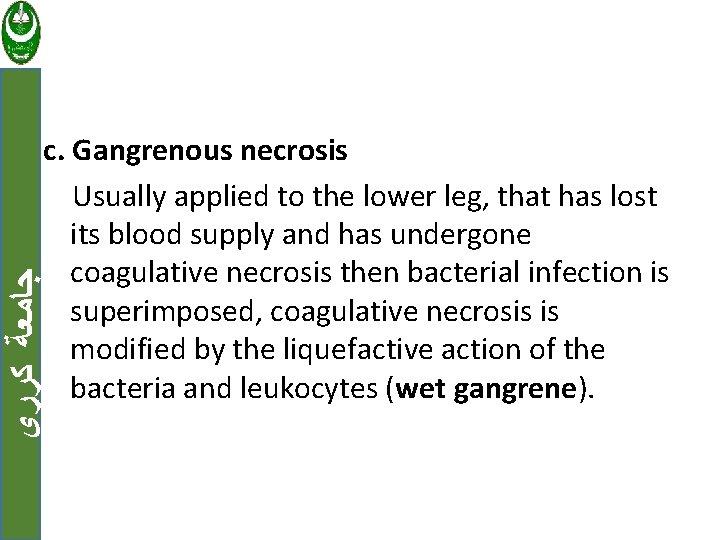
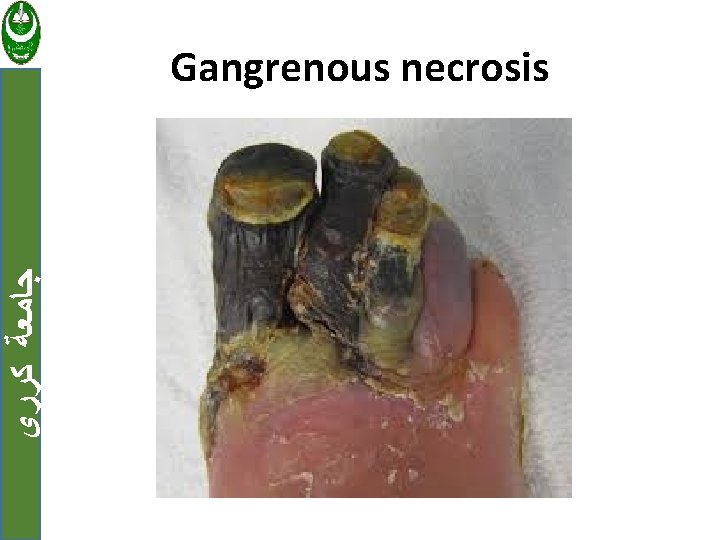
ﺟﺎﻣﻌﺔ ﻛﺮﺭﻱ c. Gangrenous necrosis Usually applied to the lower leg, that has lost its blood supply and has undergone coagulative necrosis then bacterial infection is superimposed, coagulative necrosis is modified by the liquefactive action of the bacteria and leukocytes (wet gangrene).

ﺟﺎﻣﻌﺔ ﻛﺮﺭﻱ Gangrenous necrosis

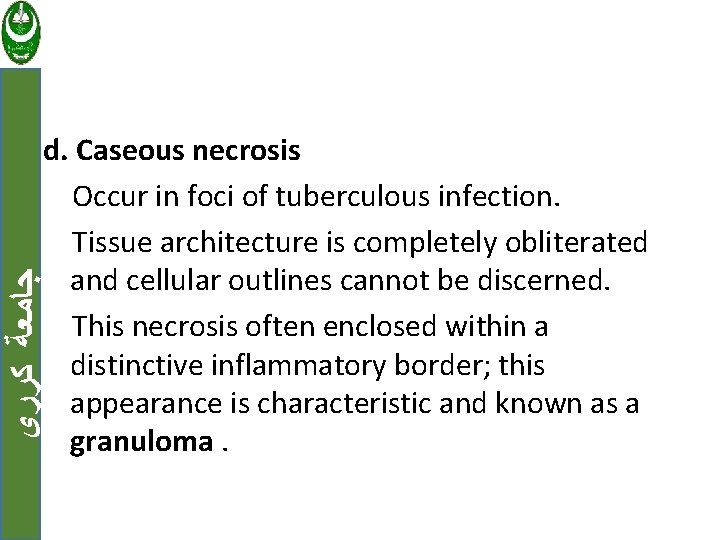
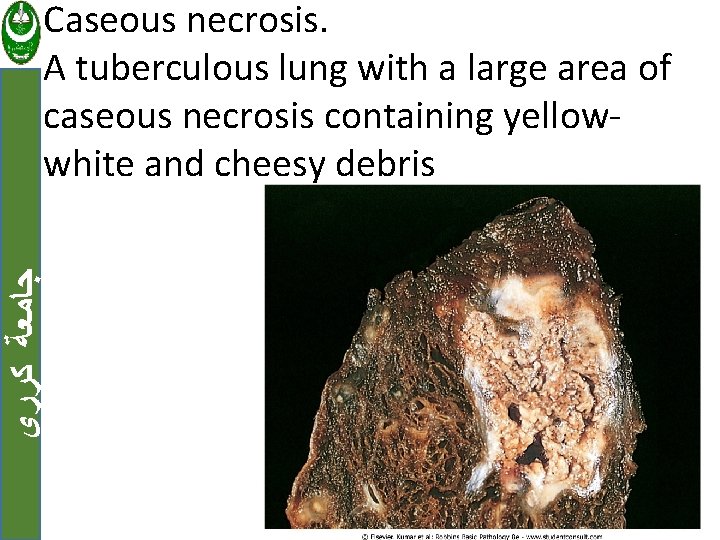
ﺟﺎﻣﻌﺔ ﻛﺮﺭﻱ d. Caseous necrosis Occur in foci of tuberculous infection. Tissue architecture is completely obliterated and cellular outlines cannot be discerned. This necrosis often enclosed within a distinctive inflammatory border; this appearance is characteristic and known as a granuloma.

ﺟﺎﻣﻌﺔ ﻛﺮﺭﻱ Caseous necrosis. A tuberculous lung with a large area of caseous necrosis containing yellowwhite and cheesy debris

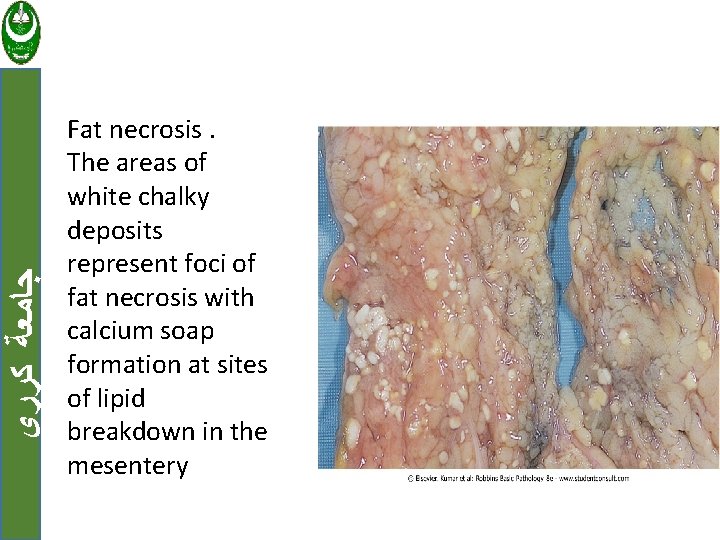
ﺟﺎﻣﻌﺔ ﻛﺮﺭﻱ e. Fat necrosis Focal areas of fat destruction, typically resulting from release of activated lipases into fat substance, e. g. acute pancreatitis.

ﺟﺎﻣﻌﺔ ﻛﺮﺭﻱ Fat necrosis. The areas of white chalky deposits represent foci of fat necrosis with calcium soap formation at sites of lipid breakdown in the mesentery

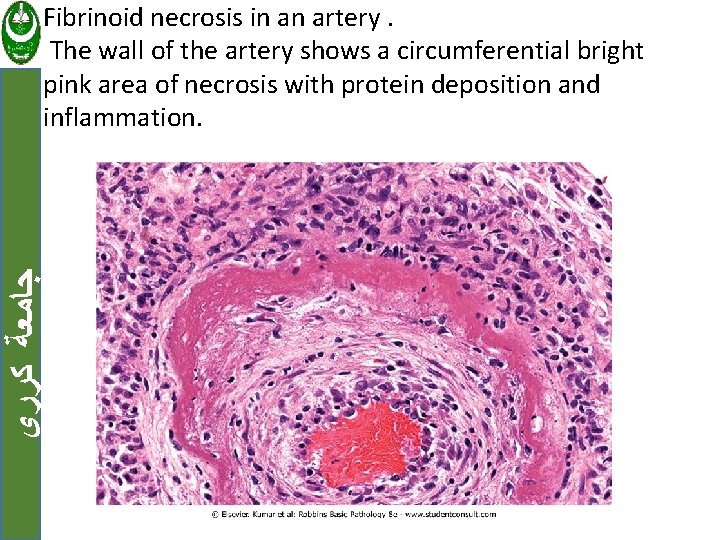
ﺟﺎﻣﻌﺔ ﻛﺮﺭﻱ f. Fibrinoid necrosis Seen in immune reactions involving blood vessels. When complexes of antigens and antibodies are deposited in the walls of arteries.

ﺟﺎﻣﻌﺔ ﻛﺮﺭﻱ Fibrinoid necrosis in an artery. The wall of the artery shows a circumferential bright pink area of necrosis with protein deposition and inflammation.

Apoptosis ﺟﺎﻣﻌﺔ ﻛﺮﺭﻱ • Is a pathway of cell death that is induced by a tightly regulated suicide program in which cells destined to die activate enzymes capable of degrading the cells' own nuclear DNA and nuclear and cytoplasmic proteins.

ﺟﺎﻣﻌﺔ ﻛﺮﺭﻱ The plasma membrane of the apoptotic cell remains intact. The cells rapidly shrink and fragment into apoptotic bodies(membrane-bound vesicles of cytosol and organelles).

ﺟﺎﻣﻌﺔ ﻛﺮﺭﻱ • The apoptotic bodies is rapidly cleared by phagocytic cell before its contents have leaked out, and therefore cell death by apoptosis does not elicit an inflammatory reaction.

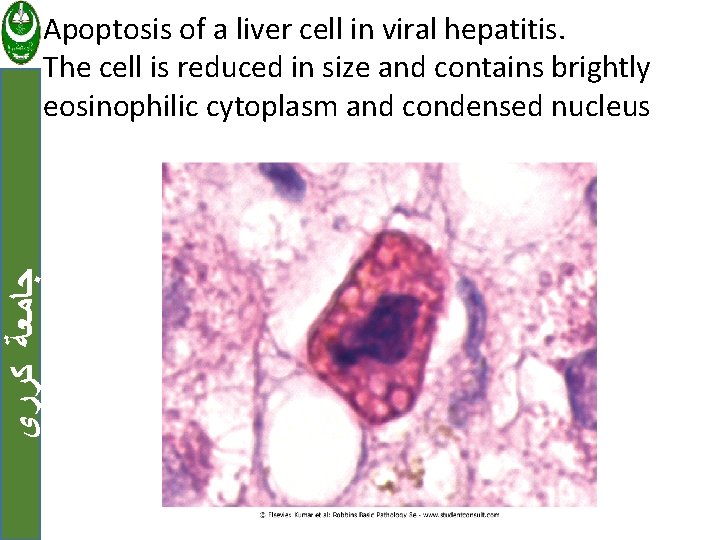
ﺟﺎﻣﻌﺔ ﻛﺮﺭﻱ Apoptosis of a liver cell in viral hepatitis. The cell is reduced in size and contains brightly eosinophilic cytoplasm and condensed nucleus

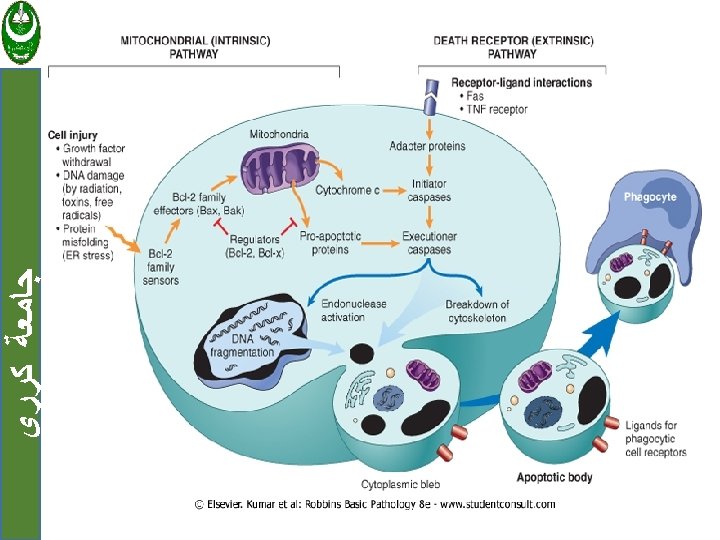
ﺟﺎﻣﻌﺔ ﻛﺮﺭﻱ The mechanism: Involved the activation of enzymes called caspases. Activated caspases cleave numerous targets, involved in activation of nucleases that degrade DNA and other enzymes that destroy nucleoproteins and cytokeletal proteins.

ﺟﺎﻣﻌﺔ ﻛﺮﺭﻱ Two distinct pathways involved on caspase activation: 1. Mitochondrial pathway. 2. Death receptor pathway.


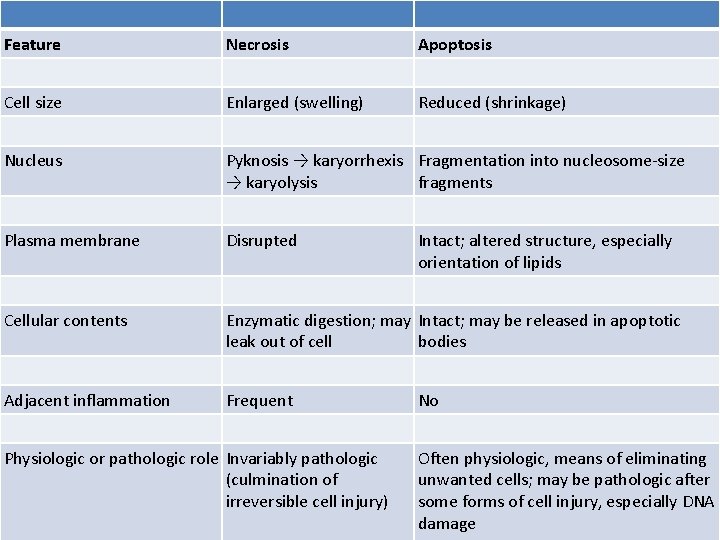
Feature Necrosis Apoptosis Cell size Enlarged (swelling) Reduced (shrinkage) Nucleus Pyknosis → karyorrhexis Fragmentation into nucleosome-size → karyolysis fragments Plasma membrane Disrupted Cellular contents Enzymatic digestion; may Intact; may be released in apoptotic leak out of cell bodies Adjacent inflammation Frequent Physiologic or pathologic role Invariably pathologic (culmination of irreversible cell injury) Intact; altered structure, especially orientation of lipids No Often physiologic, means of eliminating unwanted cells; may be pathologic after some forms of cell injury, especially DNA damage


Subcellular Responses to Injury ﺟﺎﻣﻌﺔ ﻛﺮﺭﻱ Are alterations involving only subcellular organelles. Occur in acute lethal injury, others are seen in chronic forms of cell injury, and others are adaptive responses.

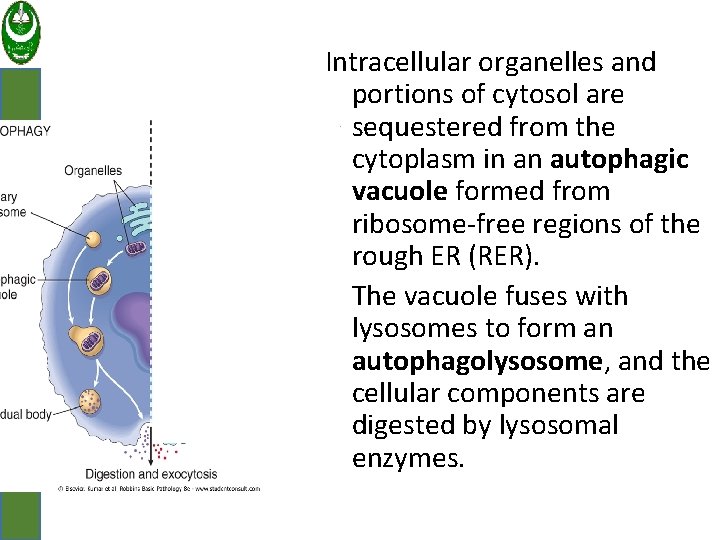
ﺟﺎﻣﻌﺔ ﻛﺮﺭﻱ 1. Autophagy: Refers to lysosomal digestion of the cell's own components. A survival mechanism in times of nutrient deprivation. The starved cell lives by eating its own contents.

ﺟﺎﻣﻌﺔ ﻛﺮﺭﻱ Intracellular organelles and portions of cytosol are sequestered from the cytoplasm in an autophagic vacuole formed from ribosome-free regions of the rough ER (RER). The vacuole fuses with lysosomes to form an autophagolysosome, and the cellular components are digested by lysosomal enzymes.

ﺟﺎﻣﻌﺔ ﻛﺮﺭﻱ 2. Induction (Hypertrophy) of Smooth ER : (SER) is involved in the metabolism of various chemicals. Cells exposed to these chemicals show hypertrophy of the ER as an adaptive response that may have important function in metabolism of this chemicals e. g; barbiturates.

ﺟﺎﻣﻌﺔ ﻛﺮﺭﻱ 3. Mitochondrial Alterations: In some nonlethal pathologic conditions, there may be alterations in the number, size, shape, and function of mitochondria. Eg; in cellular hypertrophy there is an increase in the number of mitochondria in cells.

EXAMPLES OF CELL INJURY AND NECROSIS ﺟﺎﻣﻌﺔ ﻛﺮﺭﻱ 1. Ischemia-Reperfusion Injury If cells are reversibly injured, the restoration of blood flow can result in cell recovery. Sometimes the restoration of blood flow to ischemic but viable tissues results, paradoxically, in exacerbated and accelerated injury. This is called ischemia-reperfusion injury.

ﺟﺎﻣﻌﺔ ﻛﺮﺭﻱ • it is a clinically important process that may contribute to tissue damage in myocardial and cerebral infarctions.

ﺟﺎﻣﻌﺔ ﻛﺮﺭﻱ Mechanisms of ischemia-reperfusion injury : 1. Damage may be initiated by increased generation of ROS. 2. Cellular antioxidant defense mechanisms may also be compromised by ischemia.

ﺟﺎﻣﻌﺔ ﻛﺮﺭﻱ 3. Ischemic injury is associated with inflammation, the products of activated leukocytes may cause additional tissue injury. 4. Activation of the complement system may also contribute to ischemia-reperfusion injury.

ﺟﺎﻣﻌﺔ ﻛﺮﺭﻱ 2. Chemical (Toxic) Injury Chemicals induce cell injury by: 1. Act directly by combining with a critical molecular component or cellular organelle. 2. Many other chemicals first converted to reactive toxic metabolites, which then act on target cells.

Examples of Apoptosis ﺟﺎﻣﻌﺔ ﻛﺮﺭﻱ 1. Growth Factor Deprivation: Hormone-sensitive cells deprived of the relevant hormone, lymphocytes that are not stimulated by antigens and cytokines, and neurons deprived of nerve growth factor die by apoptosis.

ﺟﺎﻣﻌﺔ ﻛﺮﺭﻱ 2. DNA Damage: Exposure of cells to radiation or chemotherapeutic agents induces DNA damage. When DNA is damaged, the p 53 protein accumulates in cells. It first arrests the cell cycle (at the G 1 phase) to allow time for repair. If the damage is too great to be repaired , p 53 triggers apoptosis.

ﺟﺎﻣﻌﺔ ﻛﺮﺭﻱ 3. Accumulation of Misfolded Proteins: During normal protein synthesis, chaperones in the ER control the proper folding of newly synthesized proteins, and misfolded polypeptides are ubiquitinated and targeted for proteolysis.

ﺟﺎﻣﻌﺔ ﻛﺮﺭﻱ • If unfolded or misfolded proteins accumulate in the ER , they induce "ER stress" that triggers a cellular responses, called the unfolded protein response

ﺟﺎﻣﻌﺔ ﻛﺮﺭﻱ • This response activates signaling pathways that increase the production of chaperones and retard protein translation, thus reducing the levels of misfolded proteins in the cell.

ﺟﺎﻣﻌﺔ ﻛﺮﺭﻱ • If this response is unable to cope with the accumulation of misfolded proteins, the result is the activation of caspases that lead to apoptosis

ﺟﺎﻣﻌﺔ ﻛﺮﺭﻱ 4. Apoptosis of Self-Reactive Lymphocytes: Lymphocytes capable of recognizing self antigens are normally produced in all individuals. If these lymphocytes encounter self antigens, the cells die by apoptosis. Failure of apoptosis of self-reactive lymphocytes is one of the causes of autoimmune diseases.