Pathogenesis and differential diagnosis of thrombotic microangiopathies Zoltn

Pathogenesis and differential diagnosis of thrombotic microangiopathies Zoltán Prohászka Research Laboratory, IIIrd Department of Medicine, Semmelweis University, Budapest prohoz@kut. sote. hu; 2016 -02 -23
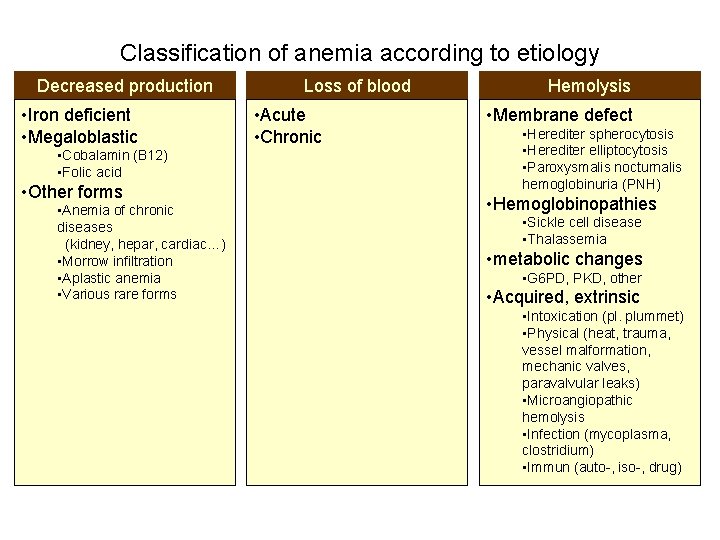
Classification of anemia according to etiology Decreased production • Iron deficient • Megaloblastic • Cobalamin (B 12) • Folic acid • Other forms • Anemia of chronic diseases (kidney, hepar, cardiac…) • Morrow infiltration • Aplastic anemia • Various rare forms Loss of blood • Acute • Chronic Hemolysis • Membrane defect • Herediter spherocytosis • Herediter elliptocytosis • Paroxysmalis nocturnalis hemoglobinuria (PNH) • Hemoglobinopathies • Sickle cell disease • Thalassemia • metabolic changes • G 6 PD, PKD, other • Acquired, extrinsic • Intoxication (pl. plummet) • Physical (heat, trauma, vessel malformation, mechanic valves, paravalvular leaks) • Microangiopathic hemolysis • Infection (mycoplasma, clostridium) • Immun (auto-, iso-, drug)
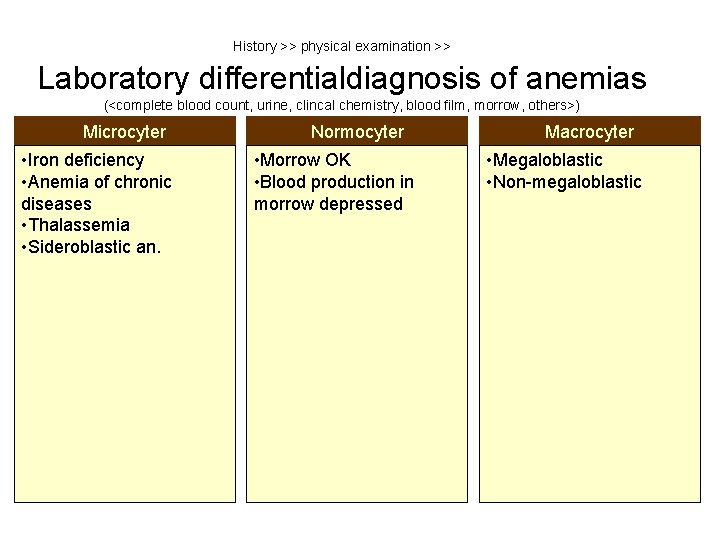
History >> physical examination >> Laboratory differentialdiagnosis of anemias (<complete blood count, urine, clincal chemistry, blood film, morrow, others>) Microcyter • Iron deficiency • Anemia of chronic diseases • Thalassemia • Sideroblastic an. Normocyter • Morrow OK • Blood production in morrow depressed Macrocyter • Megaloblastic • Non-megaloblastic
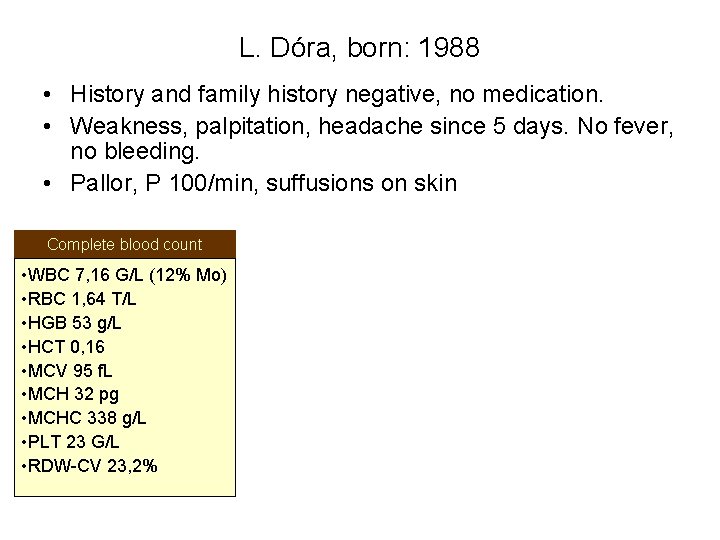
L. Dóra, born: 1988 • History and family history negative, no medication. • Weakness, palpitation, headache since 5 days. No fever, no bleeding. • Pallor, P 100/min, suffusions on skin Complete blood count • WBC 7, 16 G/L (12% Mo) • RBC 1, 64 T/L • HGB 53 g/L • HCT 0, 16 • MCV 95 f. L • MCH 32 pg • MCHC 338 g/L • PLT 23 G/L • RDW-CV 23, 2%
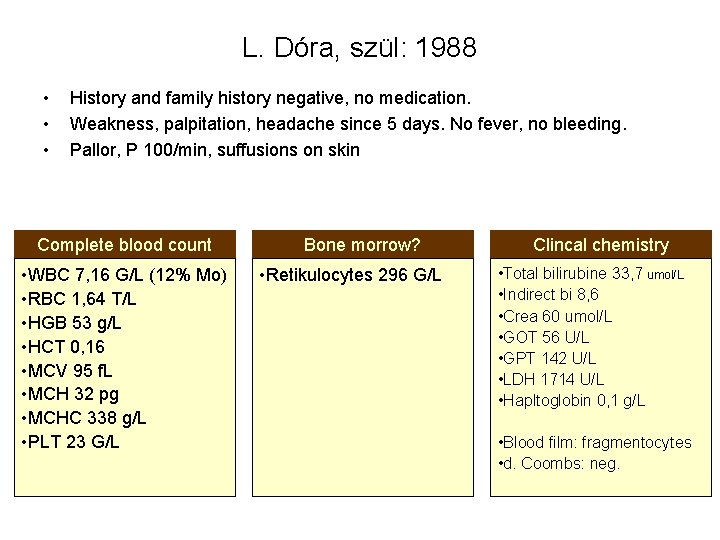
L. Dóra, szül: 1988 • • • History and family history negative, no medication. Weakness, palpitation, headache since 5 days. No fever, no bleeding. Pallor, P 100/min, suffusions on skin Complete blood count • WBC 7, 16 G/L (12% Mo) • RBC 1, 64 T/L • HGB 53 g/L • HCT 0, 16 • MCV 95 f. L • MCH 32 pg • MCHC 338 g/L • PLT 23 G/L Bone morrow? • Retikulocytes 296 G/L Clincal chemistry • Total bilirubine 33, 7 umol/L • Indirect bi 8, 6 • Crea 60 umol/L • GOT 56 U/L • GPT 142 U/L • LDH 1714 U/L • Hapltoglobin 0, 1 g/L • Blood film: fragmentocytes • d. Coombs: neg.
Complexity of the diagnosis: thrombotic microangiopathy (TMA) • Goal: the right patient should receive the right therapy in due time • Considerations on: – Timeline (acute- remission- relapse- long term management) – Therapy decisions (acute- upfront; remission- conclusive; relapseprevention) – Alternate causes (definitive TMA, probable TMA, possible TMA) – Risks of therapy (infants, gestation, peri- or post tx) – Implementation/Access to therapy (PI, PEX, eculizumab)
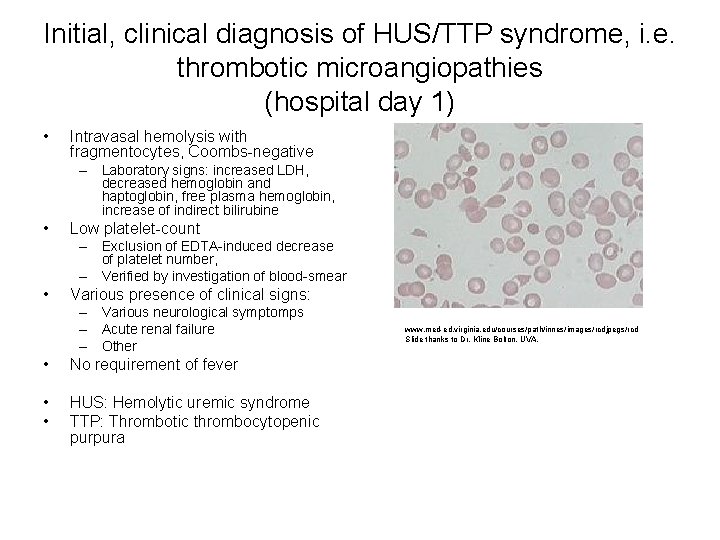
Initial, clinical diagnosis of HUS/TTP syndrome, i. e. thrombotic microangiopathies (hospital day 1) • Intravasal hemolysis with fragmentocytes, Coombs-negative – Laboratory signs: increased LDH, decreased hemoglobin and haptoglobin, free plasma hemoglobin, increase of indirect bilirubine • Low platelet-count – Exclusion of EDTA-induced decrease of platelet number, – Verified by investigation of blood-smear • Various presence of clinical signs: – Various neurological symptomps – Acute renal failure – Other • No requirement of fever • • HUS: Hemolytic uremic syndrome TTP: Thrombotic thrombocytopenic purpura www. med-ed. virginia. edu/courses/path/innes/images/rcdjpegs/rcd Slide thanks to Dr. Kline Bolton, UVA.
Initial, clinical diagnosis of HUS/TTP syndrome (hospital day 1) • Intravasal hemolysis with • fragmentocytes, Coombs-negative • – Laboratory signs: increased LDH, decreased hemoglobin and haptoglobin, free plasma hemoglobin, increase of indirect bilirubine • Low platelet-count – Exclusion of EDTA-induced decrease of platelet number, – Verified by investigation of bloodsmear • Various presence of clinical signs: – Neurological or renal • No requirement of fever (1) Besbas et al, 2006, Kidney International (2) Ariceta et al, 2009, Pediatric Nephrology Simplified classification of HUS/TTP Infection-related HUS – EHEC D+HUS ‘typical’ (1 -2)
Clinical course of thrombotic microangiopathies (HUS/TTP syndrome) Predisposition, direct cause of disease D+HUS Acute disease episode, hemolysis, thrombocytopenia Complication, outcome Shiga-like toxin producing bacteria, gastroenteritis Rarely a. HUS P-HUS TTP (Moschcowitz sy) Congenital TTP (Upshaw-Schulman sy) Secondary HUS/TTP

www. eurosurveillance. org
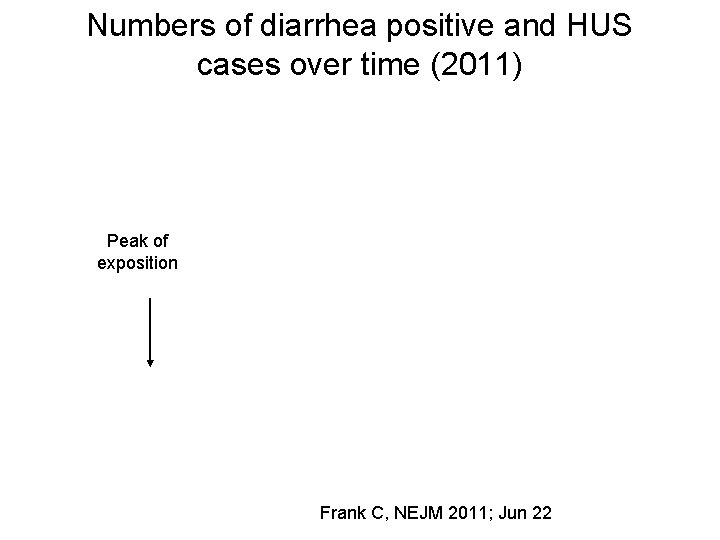
Numbers of diarrhea positive and HUS cases over time (2011) Peak of exposition Frank C, NEJM 2011; Jun 22
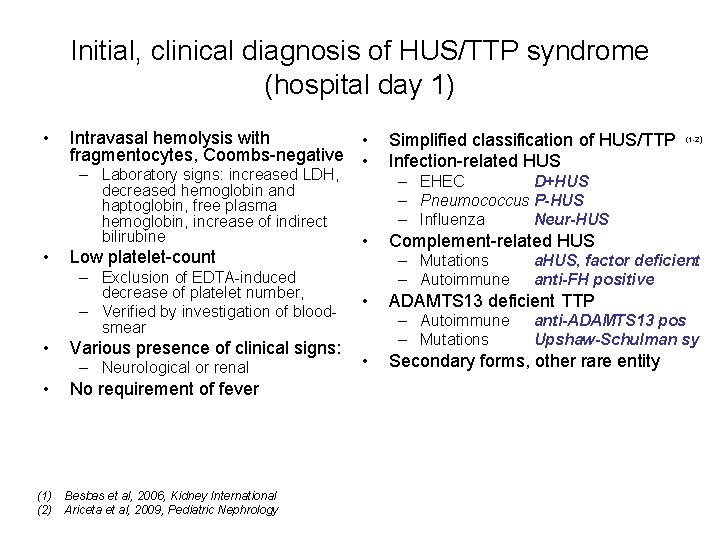
Initial, clinical diagnosis of HUS/TTP syndrome (hospital day 1) • Intravasal hemolysis with • fragmentocytes, Coombs-negative • – Laboratory signs: increased LDH, decreased hemoglobin and haptoglobin, free plasma hemoglobin, increase of indirect bilirubine • Low platelet-count – Exclusion of EDTA-induced decrease of platelet number, – Verified by investigation of bloodsmear • Various presence of clinical signs: – Neurological or renal • No requirement of fever (1) Besbas et al, 2006, Kidney International (2) Ariceta et al, 2009, Pediatric Nephrology Simplified classification of HUS/TTP Infection-related HUS (1 -2) – EHEC D+HUS – Pneumococcus P-HUS – Influenza Neur-HUS • Complement-related HUS – Mutations – Autoimmune • ADAMTS 13 deficient TTP – Autoimmune – Mutations • a. HUS, factor deficient anti-FH positive anti-ADAMTS 13 pos Upshaw-Schulman sy Secondary forms, other rare entity
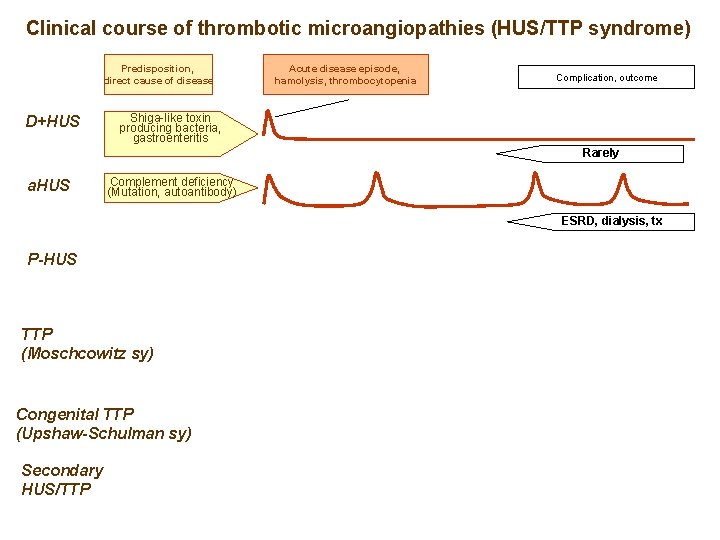
Clinical course of thrombotic microangiopathies (HUS/TTP syndrome) Predisposition, direct cause of disease D+HUS Acute disease episode, hamolysis, thrombocytopenia Complication, outcome Shiga-like toxin producing bacteria, gastroenteritis Rarely a. HUS Complement deficiency (Mutation, autoantibody) ESRD, dialysis, tx P-HUS TTP (Moschcowitz sy) Congenital TTP (Upshaw-Schulman sy) Secondary HUS/TTP
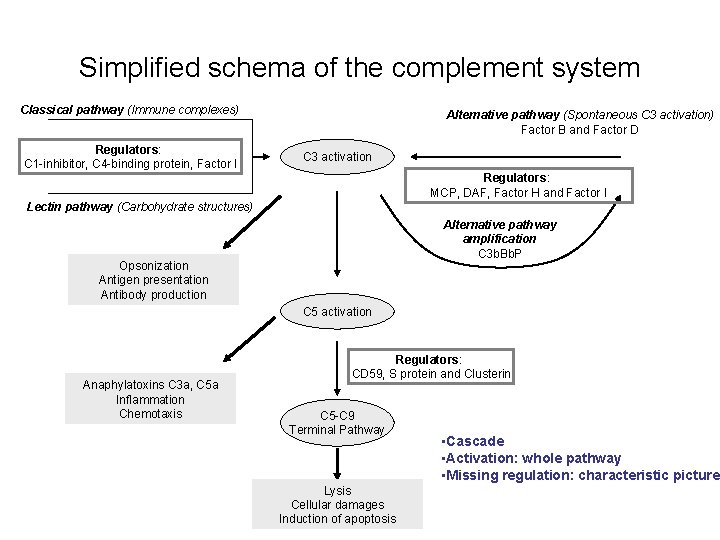
Simplified schema of the complement system Classical pathway (Immune complexes) Regulators: C 1 -inhibitor, C 4 -binding protein, Factor I Alternative pathway (Spontaneous C 3 activation) Factor B and Factor D C 3 activation Regulators: MCP, DAF, Factor H and Factor I Lectin pathway (Carbohydrate structures) Alternative pathway amplification C 3 b. Bb. P Opsonization Antigen presentation Antibody production C 5 activation Anaphylatoxins C 3 a, C 5 a Inflammation Chemotaxis Regulators: CD 59, S protein and Clusterin C 5 -C 9 Terminal Pathway Lysis Cellular damages Induction of apoptosis • Cascade • Activation: whole pathway • Missing regulation: characteristic picture
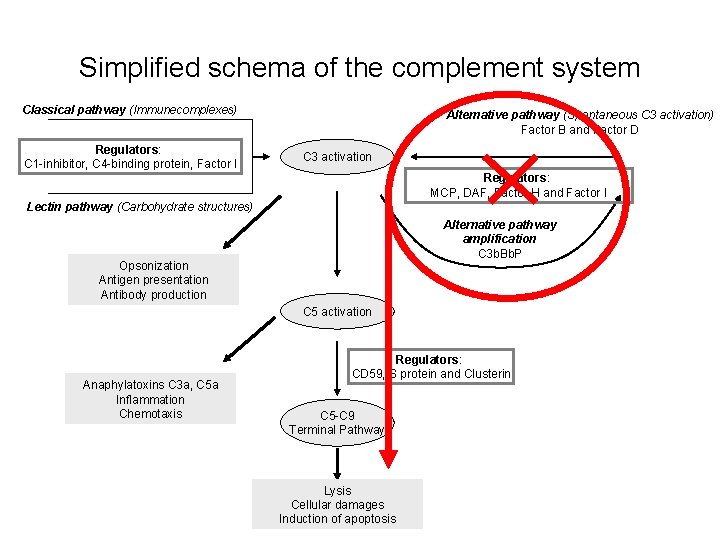
Simplified schema of the complement system Classical pathway (Immunecomplexes) Regulators: C 1 -inhibitor, C 4 -binding protein, Factor I Alternative pathway (Spontaneous C 3 activation) Factor B and Factor D C 3 activation Regulators: MCP, DAF, Factor H and Factor I Lectin pathway (Carbohydrate structures) Alternative pathway amplification C 3 b. Bb. P Opsonization Antigen presentation Antibody production C 5 activation Anaphylatoxins C 3 a, C 5 a Inflammation Chemotaxis Regulators: CD 59, S protein and Clusterin C 5 -C 9 Terminal Pathway Lysis Cellular damages Induction of apoptosis
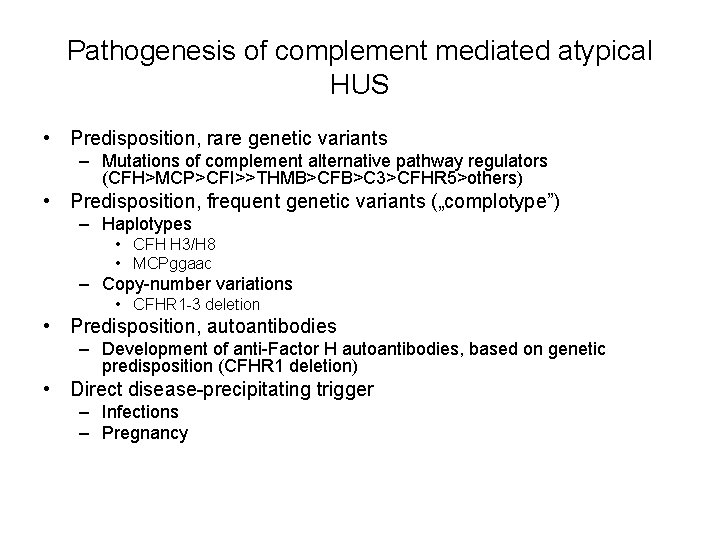
Pathogenesis of complement mediated atypical HUS • Predisposition, rare genetic variants – Mutations of complement alternative pathway regulators (CFH>MCP>CFI>>THMB>CFB>C 3>CFHR 5>others) • Predisposition, frequent genetic variants („complotype”) – Haplotypes • CFH H 3/H 8 • MCPggaac – Copy-number variations • CFHR 1 -3 deletion • Predisposition, autoantibodies – Development of anti-Factor H autoantibodies, based on genetic predisposition (CFHR 1 deletion) • Direct disease-precipitating trigger – Infections – Pregnancy
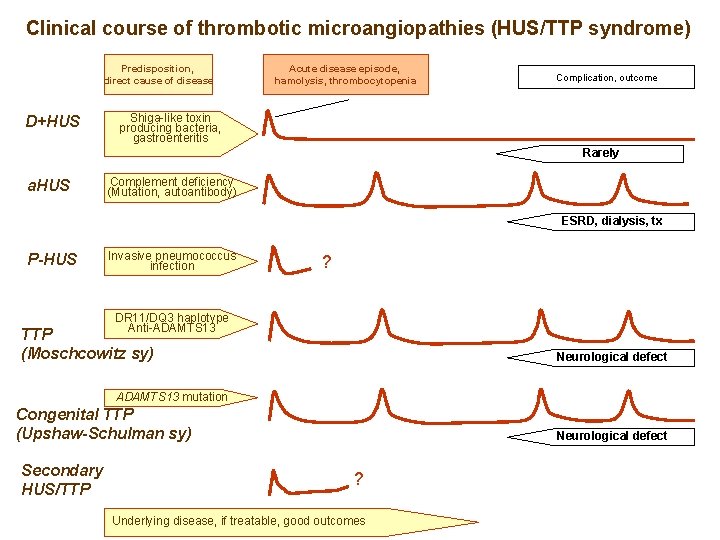
Clinical course of thrombotic microangiopathies (HUS/TTP syndrome) Predisposition, direct cause of disease D+HUS Acute disease episode, hamolysis, thrombocytopenia Complication, outcome Shiga-like toxin producing bacteria, gastroenteritis Rarely a. HUS Complement deficiency (Mutation, autoantibody) ESRD, dialysis, tx P-HUS Invasive pneumococcus infection ? DR 11/DQ 3 haplotype Anti-ADAMTS 13 TTP (Moschcowitz sy) Neurological defect ADAMTS 13 mutation Congenital TTP (Upshaw-Schulman sy) Secondary HUS/TTP Neurological defect ? Underlying disease, if treatable, good outcomes
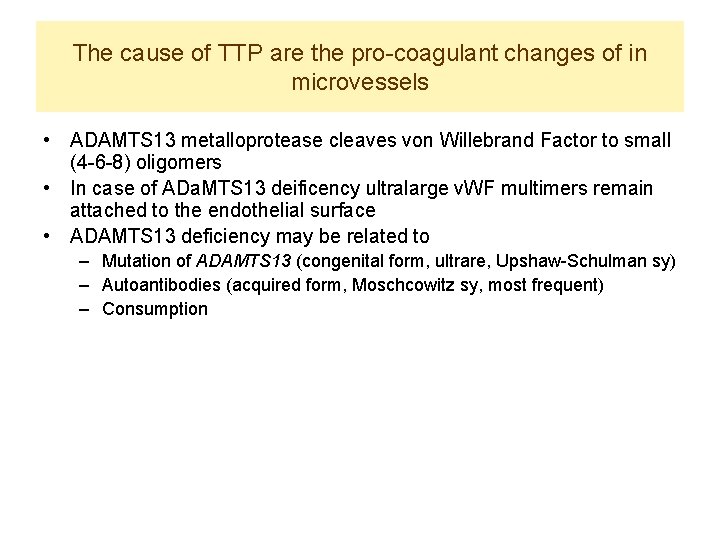
The cause of TTP are the pro-coagulant changes of in microvessels • ADAMTS 13 metalloprotease cleaves von Willebrand Factor to small (4 -6 -8) oligomers • In case of ADa. MTS 13 deificency ultralarge v. WF multimers remain attached to the endothelial surface • ADAMTS 13 deficiency may be related to – Mutation of ADAMTS 13 (congenital form, ultrare, Upshaw-Schulman sy) – Autoantibodies (acquired form, Moschcowitz sy, most frequent) – Consumption
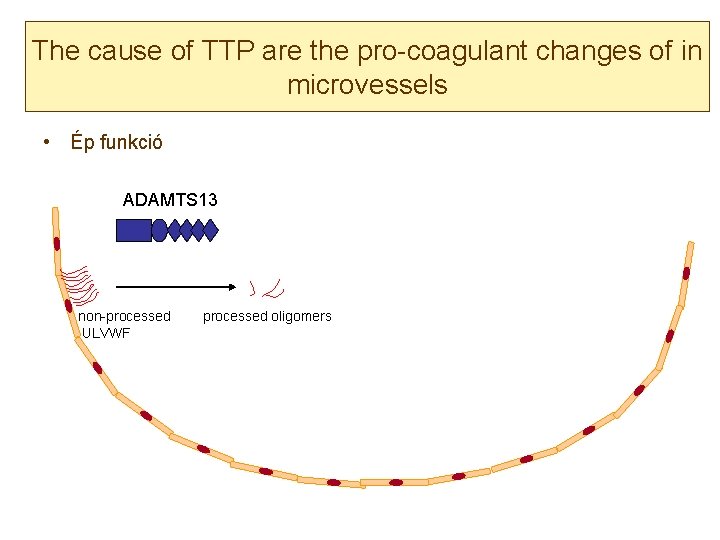
The cause of TTP are the pro-coagulant changes of in microvessels • Ép funkció ADAMTS 13 non-processed ULVWF processed oligomers
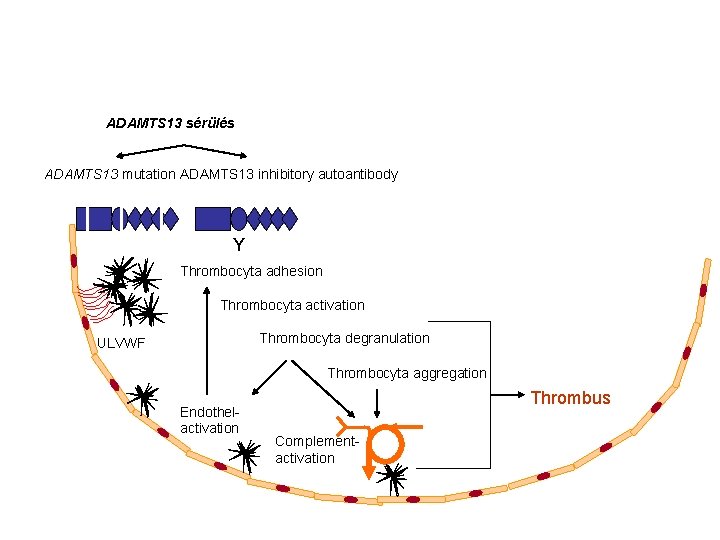
ADAMTS 13 sérülés ADAMTS 13 mutation ADAMTS 13 inhibitory autoantibody Y Thrombocyta adhesion Thrombocyta activation Thrombocyta degranulation ULVWF Thrombocyta aggregation Endothelactivation Thrombus Complementactivation
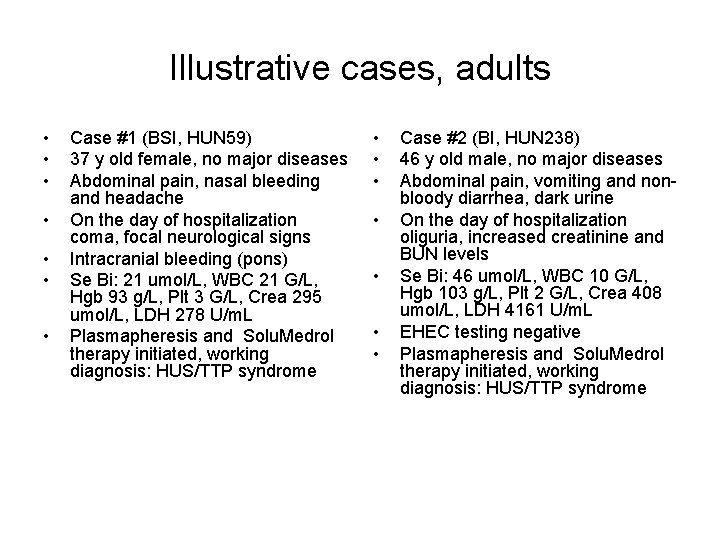
Illustrative cases, adults • • Case #1 (BSI, HUN 59) 37 y old female, no major diseases Abdominal pain, nasal bleeding and headache On the day of hospitalization coma, focal neurological signs Intracranial bleeding (pons) Se Bi: 21 umol/L, WBC 21 G/L, Hgb 93 g/L, Plt 3 G/L, Crea 295 umol/L, LDH 278 U/m. L Plasmapheresis and Solu. Medrol therapy initiated, working diagnosis: HUS/TTP syndrome • • Case #2 (BI, HUN 238) 46 y old male, no major diseases Abdominal pain, vomiting and nonbloody diarrhea, dark urine On the day of hospitalization oliguria, increased creatinine and BUN levels Se Bi: 46 umol/L, WBC 10 G/L, Hgb 103 g/L, Plt 2 G/L, Crea 408 umol/L, LDH 4161 U/m. L EHEC testing negative Plasmapheresis and Solu. Medrol therapy initiated, working diagnosis: HUS/TTP syndrome
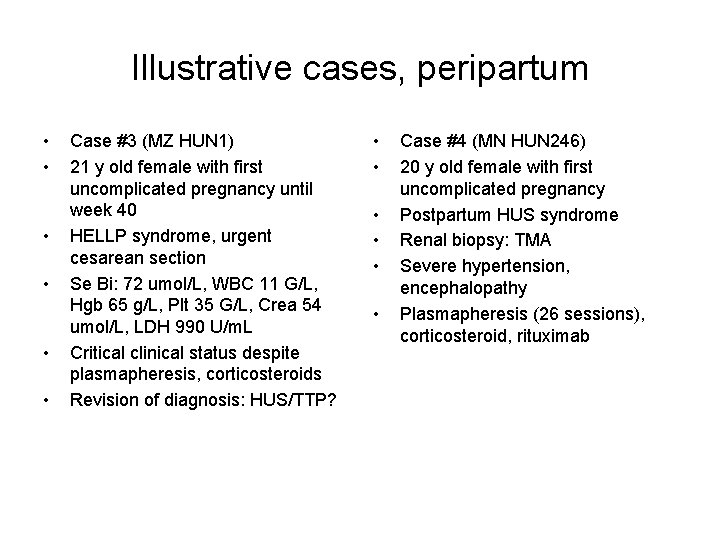
Illustrative cases, peripartum • • • Case #3 (MZ HUN 1) 21 y old female with first uncomplicated pregnancy until week 40 HELLP syndrome, urgent cesarean section Se Bi: 72 umol/L, WBC 11 G/L, Hgb 65 g/L, Plt 35 G/L, Crea 54 umol/L, LDH 990 U/m. L Critical clinical status despite plasmapheresis, corticosteroids Revision of diagnosis: HUS/TTP? • • • Case #4 (MN HUN 246) 20 y old female with first uncomplicated pregnancy Postpartum HUS syndrome Renal biopsy: TMA Severe hypertension, encephalopathy Plasmapheresis (26 sessions), corticosteroid, rituximab
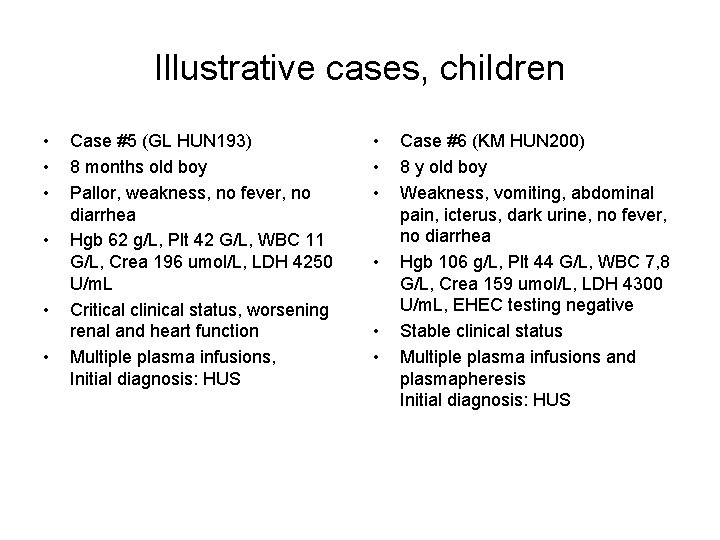
Illustrative cases, children • • • Case #5 (GL HUN 193) 8 months old boy Pallor, weakness, no fever, no diarrhea Hgb 62 g/L, Plt 42 G/L, WBC 11 G/L, Crea 196 umol/L, LDH 4250 U/m. L Critical clinical status, worsening renal and heart function Multiple plasma infusions, Initial diagnosis: HUS • • • Case #6 (KM HUN 200) 8 y old boy Weakness, vomiting, abdominal pain, icterus, dark urine, no fever, no diarrhea Hgb 106 g/L, Plt 44 G/L, WBC 7, 8 G/L, Crea 159 umol/L, LDH 4300 U/m. L, EHEC testing negative Stable clinical status Multiple plasma infusions and plasmapheresis Initial diagnosis: HUS
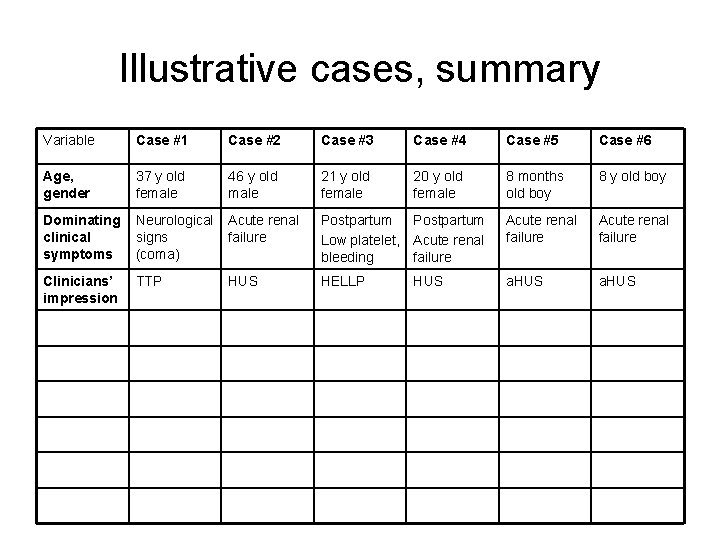
Illustrative cases, summary Variable Case #1 Case #2 Case #3 Case #4 Case #5 Case #6 Age, gender 37 y old female 46 y old male 21 y old female 20 y old female 8 months old boy 8 y old boy Dominating clinical symptoms Neurological signs (coma) Acute renal failure Postpartum Low platelet, Acute renal bleeding failure Acute renal failure Clinicians’ impression TTP HUS HELLP a. HUS HUS

Initial, life-threatening therapy • PE with FFP is still the most effective treatment available for TTP (1) – Rituximab (or cyclophosphamide) is indicated for patients with chronic relapsing disease (autoimmune) – Anti-platelet therapy and immunosuppression may also be considered • For HUS, most of the RCTs focused on typical HUS and showed that supportive therapy including dialysis is still the most effective treatment (1) • The „Guideline for the investigation and initial therapy of diarrheanegative hemolytic uremic syndrome” article indicates initiation of PE with FFP in the first 24 hours (2) – Eculizumab can be considered for proven cases of complementmediated a. HUS – Pulse cyclophosphamid and rituximab can be considered for anti-FH autoantibody mediated a. HUS (1) Michael et al, The Cochrane Library 2009, Issue 1 (2) Ariceta et al, 2009, Ped Nephrol
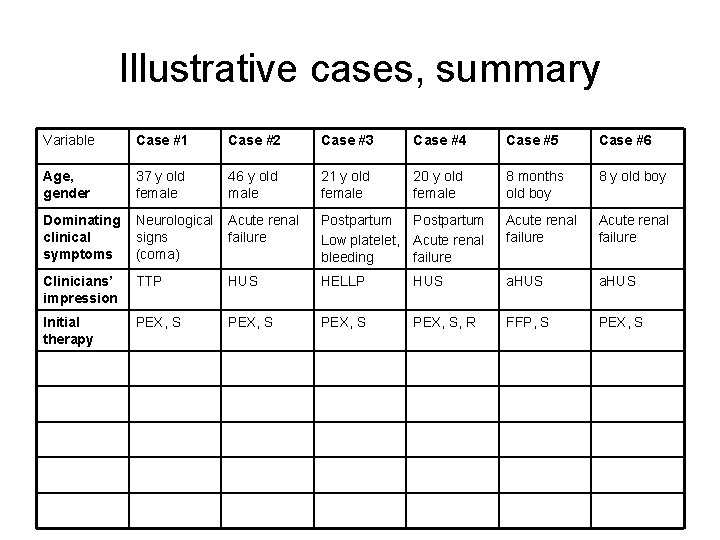
Illustrative cases, summary Variable Case #1 Case #2 Case #3 Case #4 Case #5 Case #6 Age, gender 37 y old female 46 y old male 21 y old female 20 y old female 8 months old boy 8 y old boy Dominating clinical symptoms Neurological signs (coma) Acute renal failure Postpartum Low platelet, Acute renal bleeding failure Acute renal failure Clinicians’ impression TTP HUS HELLP HUS a. HUS Initial therapy PEX, S, R FFP, S PEX, S
Therapeutic dilemma on day ~3 -6 • Judgment of initial response to plasma therapy, steroids • Results of initial laboratory testing, exclusion of: – – Sepsis, DIC Anti-phospholipid syndrome and SLE Other causes of hemolysis and low platelet numbers Secondary HUS/TTP
Therapeutic dilemma on day ~3 -6 • • Judgment of initial response to plasma therapy, steroids Results of initial laboratory testing, exclusion of: – – • Sepsis, DIC Anti-phospholipid syndrome and SLE Other causes of hemolysis and low platelet numbers Secondary HUS/TTP Where next with HUS/TTP on hospital day 3 -6? Failing or insufficient No alternative diagnosis – Is there a reliable laboratory test to classify patients into the following groups in a short time? • D+HUS • a. HUS (factor deficient or autoimmune) • TTP (ADAMTS 13 deficient with or without autoantibodies) – How to decide? • Intensify immunosuppression (Cy, R) for autoimmune disease (Ab+ a. HUS, Ab+TTP)? • Intensify factor supply/ targeted inhibition of complement (factor deficient TTP or a. HUS)?
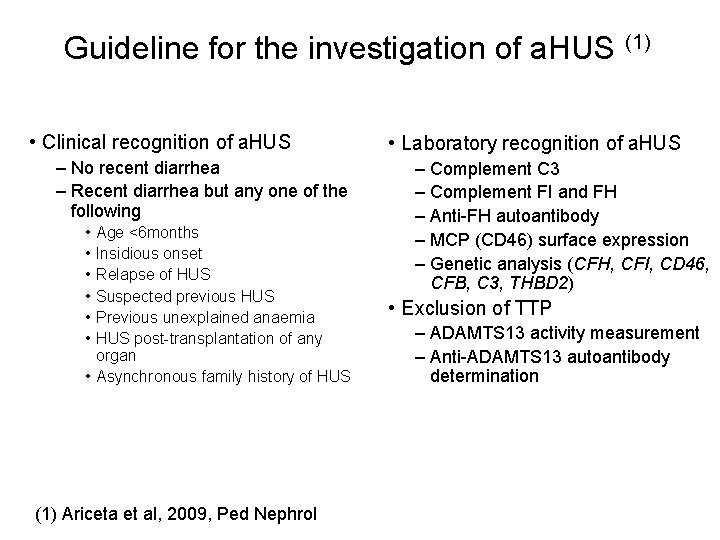
Guideline for the investigation of a. HUS (1) • Clinical recognition of a. HUS – No recent diarrhea – Recent diarrhea but any one of the following • • • Age <6 months Insidious onset Relapse of HUS Suspected previous HUS Previous unexplained anaemia HUS post-transplantation of any organ • Asynchronous family history of HUS (1) Ariceta et al, 2009, Ped Nephrol • Laboratory recognition of a. HUS – Complement C 3 – Complement FI and FH – Anti-FH autoantibody – MCP (CD 46) surface expression – Genetic analysis (CFH, CFI, CD 46, CFB, C 3, THBD 2) • Exclusion of TTP – ADAMTS 13 activity measurement – Anti-ADAMTS 13 autoantibody determination
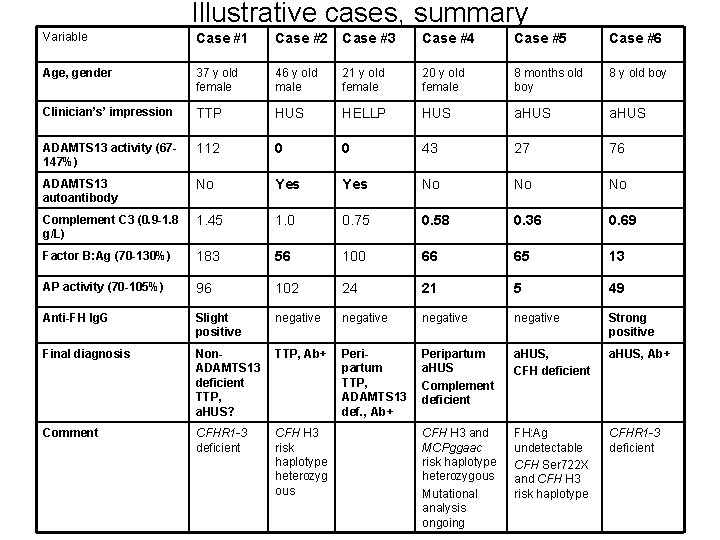
Illustrative cases, summary Variable Case #1 Case #2 Case #3 Case #4 Case #5 Case #6 Age, gender 37 y old female 46 y old male 21 y old female 20 y old female 8 months old boy 8 y old boy Clinician’s’ impression TTP HUS HELLP HUS a. HUS ADAMTS 13 activity (67147%) 112 0 0 43 27 76 ADAMTS 13 autoantibody No Yes No No No Complement C 3 (0. 9 -1. 8 g/L) 1. 45 1. 0 0. 75 0. 58 0. 36 0. 69 Factor B: Ag (70 -130%) 183 56 100 66 65 13 AP activity (70 -105%) 96 102 24 21 5 49 Anti-FH Ig. G Slight positive negative Strong positive Final diagnosis Non. ADAMTS 13 deficient TTP, a. HUS? TTP, Ab+ Peripartum TTP, ADAMTS 13 def. , Ab+ Peripartum a. HUS Complement deficient a. HUS, CFH deficient a. HUS, Ab+ Comment CFHR 1 -3 deficient CFH H 3 risk haplotype heterozyg ous CFH H 3 and MCPggaac risk haplotype heterozygous Mutational analysis ongoing FH: Ag undetectable CFH Ser 722 X and CFH H 3 risk haplotype CFHR 1 -3 deficient
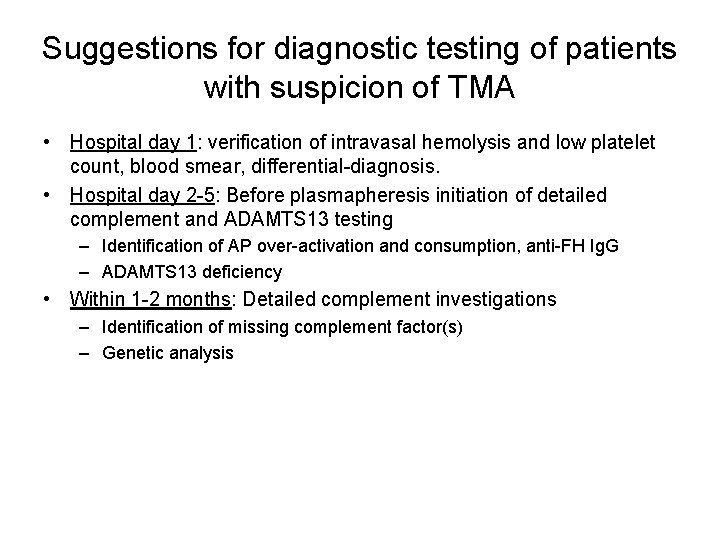
Suggestions for diagnostic testing of patients with suspicion of TMA • Hospital day 1: verification of intravasal hemolysis and low platelet count, blood smear, differential-diagnosis. • Hospital day 2 -5: Before plasmapheresis initiation of detailed complement and ADAMTS 13 testing – Identification of AP over-activation and consumption, anti-FH Ig. G – ADAMTS 13 deficiency • Within 1 -2 months: Detailed complement investigations – Identification of missing complement factor(s) – Genetic analysis

Coworkers of the Füst György Komplement Diagnosztikai Laboratórium www. kutlab. hu CEE Roundtable discussion, Zoltan Prohaszka 23 -10 -2014 Photo: Kata Tolnai
- Slides: 32