Pathogen Inactivation Technologies Where Are We Now Dana














- Slides: 25

Pathogen Inactivation Technologies: Where Are We Now? Dana Devine Chief Scientist October 25, 2018

Overview • Pathogen inactivation (PI) technologies – what are they? Who uses them? • Laboratory investigations of the effect of PI on platelet function • Clinical assessment of platelet function after PI • Where do we go from here? 2

Pathogen Inavctivation: The Technology

Pathogen Inactivation • Pathogen Inactivation is not a new concept: • Principles adopted from pathogen inactivation of plasma protein derivatives • Became available in Europe for platelets and plasma in the early 2000’s and has since gained rapid acceptance and adoption. • Two technologies are available commercially: • Intercept by Cerus, since 2002 • Mirasol by Terumo BCT, since 2007 • One in clinical trials: • Theraflex by Macopharma 4

Pathogen Inactivation of Platelets, Plasma and RBCs PI technologies • Effective against most transfusion transmissible agents • Dose must balance killing pathogens with killing the transfusion cells • Risk mitigation must consider both infectious risks and risks to product efficacy 5

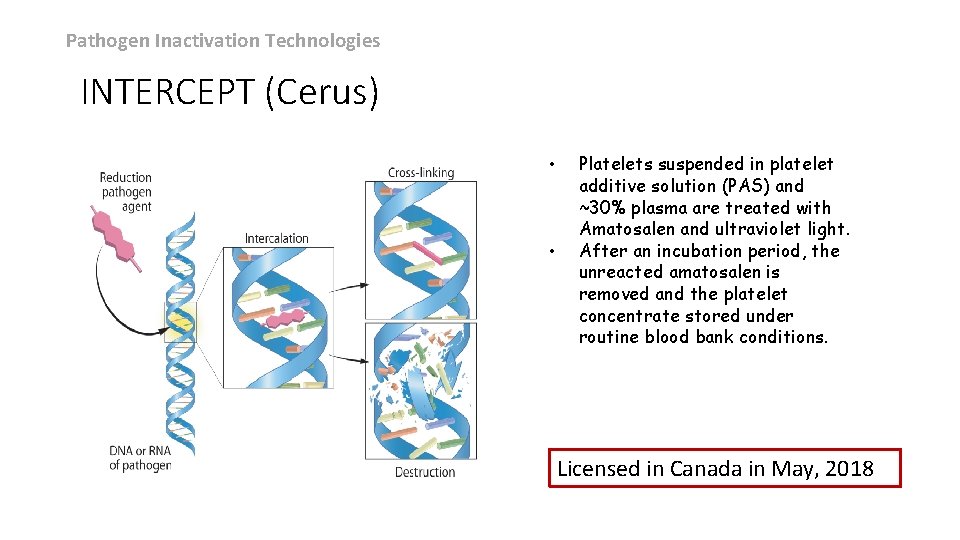
Pathogen Inactivation Technologies INTERCEPT (Cerus) • • Platelets suspended in platelet additive solution (PAS) and ~30% plasma are treated with Amatosalen and ultraviolet light. After an incubation period, the unreacted amatosalen is removed and the platelet concentrate stored under routine blood bank conditions. Licensed in Canada in May, 2018

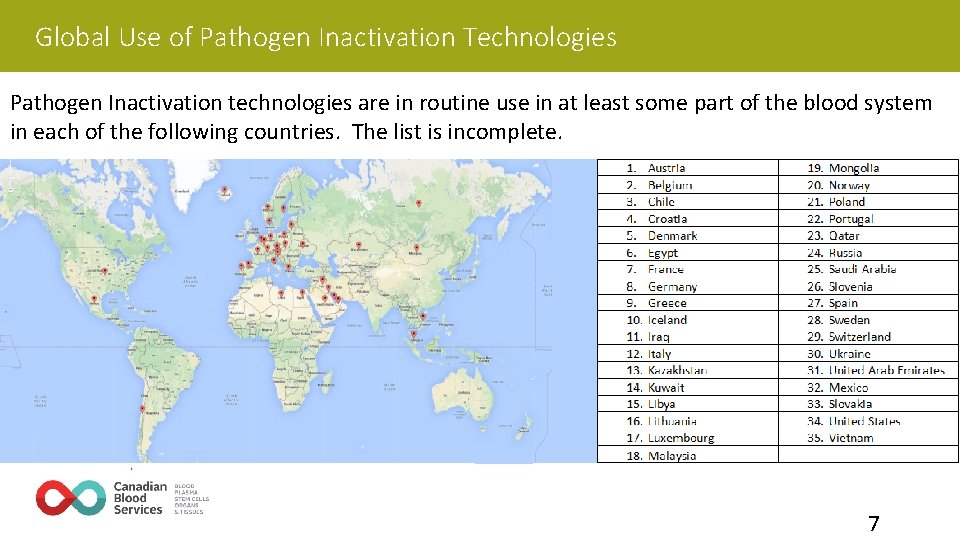
Global Use of Pathogen Inactivation Technologies Pathogen Inactivation technologies are in routine use in at least some part of the blood system in each of the following countries. The list is incomplete. 7

Laboratory Assessment of Platelet Function Following PI Treatment

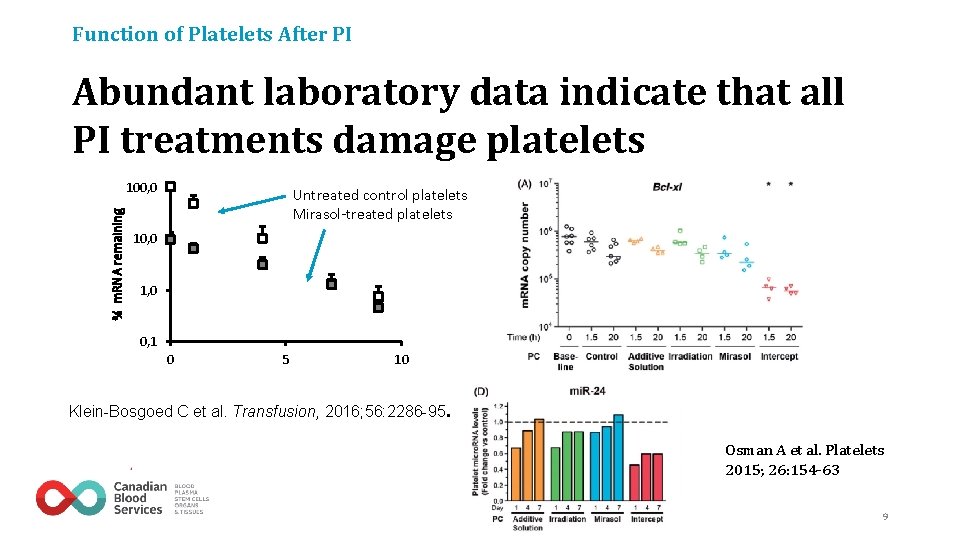
Function of Platelets After PI Abundant laboratory data indicate that all PI treatments damage platelets % m. RNA remaining 100, 0 Untreated control platelets Mirasol-treated platelets 10, 0 1, 0 0, 1 0 5 10 Klein-Bosgoed C et al. Transfusion, 2016; 56: 2286 -95. Osman A et al. Platelets 2015; 26: 154 -63 9

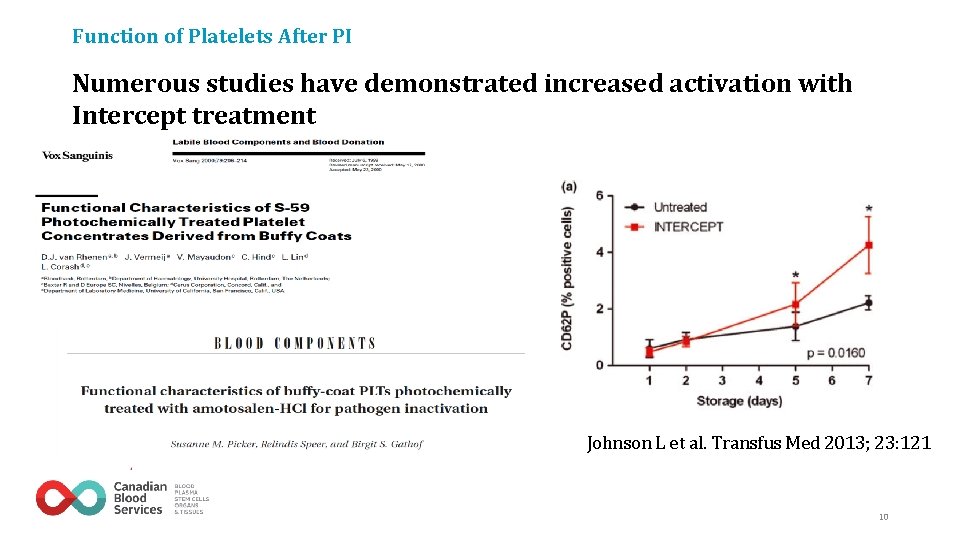
Function of Platelets After PI Numerous studies have demonstrated increased activation with Intercept treatment Johnson L et al. Transfus Med 2013; 23: 121 10

Clinical Assessment of PItreated Platelet Function

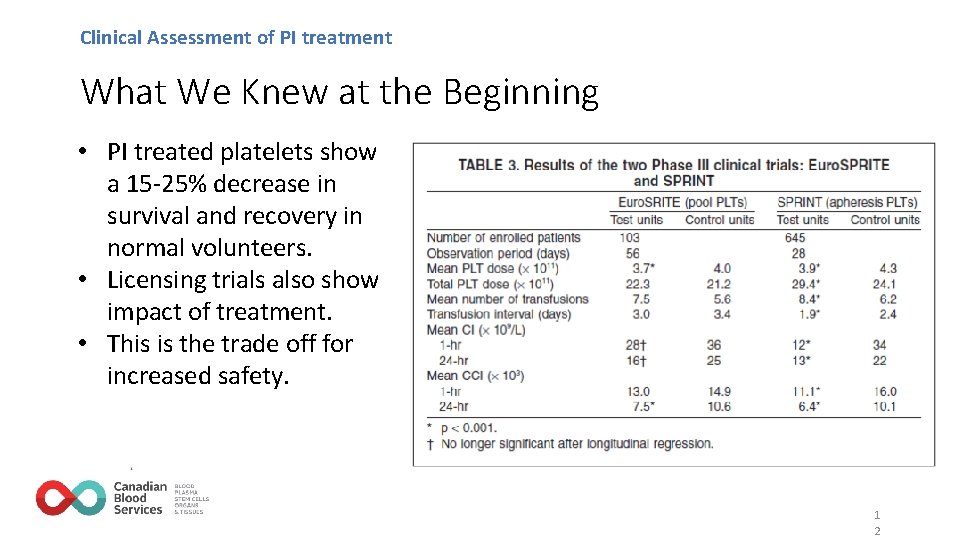
Clinical Assessment of PI treatment What We Knew at the Beginning • PI treated platelets show a 15 -25% decrease in survival and recovery in normal volunteers. • Licensing trials also show impact of treatment. • This is the trade off for increased safety. 1 2

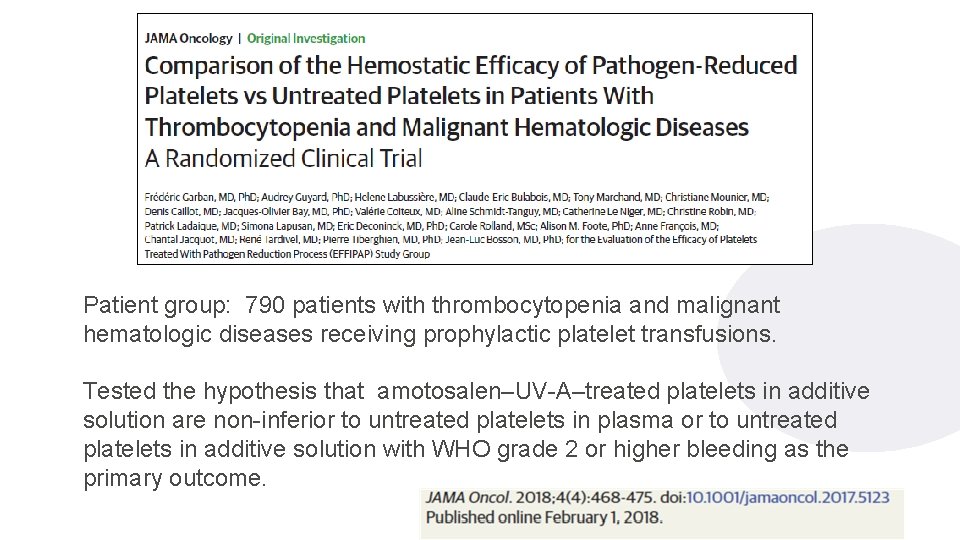
Clinical Assessment of Platelets Treated with PI Estcourt LJ et al. JAMA Onc 2018; 4: 571 -2. 1 3

Patient group: 790 patients with thrombocytopenia and malignant hematologic diseases receiving prophylactic platelet transfusions. Tested the hypothesis that amotosalen–UV-A–treated platelets in additive solution are non-inferior to untreated platelets in plasma or to untreated platelets in additive solution with WHO grade 2 or higher bleeding as the primary outcome. 14/02/2022 14

EFFIPAP Study of Intercept Platelets Platelet damage from pathogen inactivation In the EFFIPAP study with a primary bleeding endpoint, • Non-inferiority was not achieved when comparing Intercept pathogenreduced platelets in additive solution to untreated platelets in plasma. • Non-inferiority was achieved when comparing Intercept pathogen-reduced platelets in additive solution to untreated platelets in additive solution. • Pathogen reduction technology in combination with PAS-C could be associated with reduced hemostatic efficacy compared with untreated platelets stored in plasma. 14/02/2022 1 5

Pathogen Reduction Evaluation & Predictive Analytical Rating Score (PREPARe. S) • A prospective, randomized, single-blinded, multicenter noninferiority trial for the side by side evaluation of Mirasol-treated and standard of care pooled platelet products (buffy-coat platelets in plasma) in 469 hemato-oncological patients. • Initiated in the Netherlands in November 2010. Expanded to Canada and Norway. Completed recruitment April 30, 2016. Results published last month in BLOOD (van der Meer P et al. Blood, May 17, 2018 doi: 10. 1182/blood-2018 -02 -831289)

Pathogen Reduction Evaluation & Predictive Analytical Rating Score (PREPARe. S) • Pathogen-inactivated platelets were non inferior in preventing bleeding only in intention-to-treat analysis, but not in the per protocol analysis. • In contrast to animal models, alloimmunization could not be prevented when using pathogen-inactivated platelets.

What About Patients Who Are Actively Bleeding?

PI Treated Platelets in Trauma Patients Use of PI treated blood in massive transfusion S PI of blood components (platelets, plasma, RBCs or whole blood) reduces pathogen risk but also reduces product quality and/or circulation time. SThe reduction in blood component potency has been postulated to present greater risk than benefit in countries with low risk of transfusion transmitted infections. Hess et al, Transfusion 2016; 56: 1236 -41 1 9

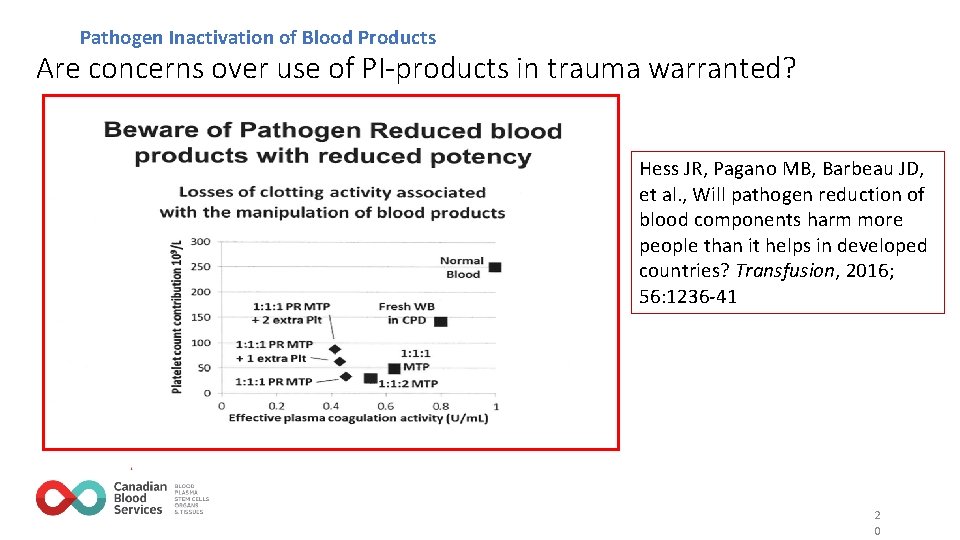
Pathogen Inactivation of Blood Products Are concerns over use of PI-products in trauma warranted? Hess JR, Pagano MB, Barbeau JD, et al. , Will pathogen reduction of blood components harm more people than it helps in developed countries? Transfusion, 2016; 56: 1236 -41 2 0

Pathogen Inactivation of Blood Products Descriptive Studies Do Not Identify a Significant Problem with PI treated Platelets in Bleeding Patients…. We need to see publications of the recent clinical studies and await the results of new studies! 2 1

Remaining Hurdles for Pathogen Inactivation Technologies Issues to Resolve on the Path to Adoption • How do we adapt transfusion practice to minimize quality impact of PI technologies? • Clinical trial data are limited. Studies are expensive to conduct and take many years to complete. • Cost challenges for many blood systems. • Consider ways to adapt the technologies (e. g. , Karolinska double platelet treatment). • Impact of PI may be greatest in countries that have least ability to pay for the technology. 2 2

Pathogen Inactivation Why is this technology of interest in Canada? • Increase the safety of the blood supply from infectious agents, even though our blood supply is very safe. • Improve our state of readiness should a new pathogen emerge that threatens blood safety for which no test is available. • Both Cerus and Terumo. BCT are working to license PI that can be applied to RBC or to whole blood. Clinical trials are underway for both systems. Design of hardware needs to be improved for use in high throughput processing environments. 2 3

Pathogen Inactivation Questions to Consider • Is the benefit of increased safety from known or unknown pathogens worth the small reduction in platelet transfusion efficacy? • As a risk management preparedness policy, does it make sense for Canadian Blood Services to introduce PI technology for at least some of the platelet inventory? • If we do a staged implementation (eg one pilot site) how should we manage the distribution of that inventory? • Are there other questions that we need to ask about PI technology use in Canada? 2 4

Visit blood. ca