Passive film growth on titanium alloys Physicochemical and



![Sources of oxygen 1. A membrane-associated nicotinamide adenine nucleotide (NADP[H]), when bound to lactic Sources of oxygen 1. A membrane-associated nicotinamide adenine nucleotide (NADP[H]), when bound to lactic](https://slidetodoc.com/presentation_image/23a1ddd4ecb4b8af259b7b835ed8cfd4/image-4.jpg)









































- Slides: 66

Passive film growth on titanium alloys : Physicochemical and biologic considerations Theodore Eliades Int. J. Oral Maxillofac. Implants 1997; 12: 621 -627

Implant surface particles and host response 1. When exposed to host tissue, circulating neutrophils and/or monocytes are recruited from the intravascular compartment to the location of exposure. 2. Upon recognition, neutrophils and monocytes experience a “respiratory burst”, during which an almost 20 -fold oxygen consumption by the cells is observed. Theodore Eliades, JOMI 1997

Implant surface particles and host response 3. There is convincing evidence that the consequent increase in oxygen secretion by these cells is mainly the result of this initial respiratory activity. 4. In addition, polymorphonuclear (PMN) cells have been found to secrete superoxide (O 2 -) and hydrogen peroxide (H 2 O 2) upon activation induced by several stimuli, including immunoglobulins and opsonized bacteria, among others. Theodore Eliades, JOMI 1997
![Sources of oxygen 1 A membraneassociated nicotinamide adenine nucleotide NADPH when bound to lactic Sources of oxygen 1. A membrane-associated nicotinamide adenine nucleotide (NADP[H]), when bound to lactic](https://slidetodoc.com/presentation_image/23a1ddd4ecb4b8af259b7b835ed8cfd4/image-4.jpg)
Sources of oxygen 1. A membrane-associated nicotinamide adenine nucleotide (NADP[H]), when bound to lactic dehydrogenase, reacts with O 2 - at a rate of 3. 6× 104 mol/L per second. 2. As a result, oxidation of NADH occurs, yielding an NAD radical, which in turn reduce can reduce O 2 to O 2 -5. 3. This form of enzymic oxidation is dependent on p. H, PO 2, and substrate concentration. Theodore Eliades, JOMI 1997

Sources of oxygen 4. Also, epinephrine, lipid peroxides, catecholamines, and oxyhemoglobin have been shown to represent supporting evidence of their reactivity when oxygen is present. 5. Auto-oxidations of this kind are chain reactions in which oxygen can serve both as initiator and chain propagator. 6. Subcellular organelles (mitochondria, microsomes, nuclei) have also been found to be capable of generating O 2 -. Theodore Eliades, JOMI 1997

Reactive oxygen derivatives 1. Several forms of reactive oxygen derivatives (ROD) have been identified in the phagocytic cell. 2. These include singlet oxygen (1 O 2), hydroxy peroxide (H 2 O 2), hydroxyl radical (OH¤ ), and hypochlorous acid (HOCl), with the three latter reactants predominating in the activated cell. Theodore Eliades, JOMI 1997

Reactive oxygen derivatives 3. Studies have shown that the ability of monocytes to produce O 2 - is time-dependent and diminishes as the inflammatory response progresses, reaching zero levels after a period of 2 weeks. 4. The fact that at the early stages of inflammation monocytes can produce increased amounts of oxygen, taken along with the differentiation of these cells at later stages, suggests that oxygen production apart from time also depends upon the state of activation and the extent of cell differentiation. Theodore Eliades, JOMI 1997

Reactive oxygen derivatives 5. The bactericidal and microbicidal action of ROD mainly results from H 2 O 2 and its complexes formed with ascorbic acid or metals. 6. The most potent action of these complexes occurs following reaction of the superoxide with myeloperoxidase and halide. 7. This effect might account for the dramatically low rate of infection occurrence associated with material implantation. Theodore Eliades, JOMI 1997

The effect of ROD on the implant material surface properties : hydrogen peroxide 1. Hydrogen peroxide is formed as a product of both enzymic and spontaneous dismutation, and thus Ti and H 2 O 2 may react as proposed by the general mechanism. 2. When H 2 O 2 and Ti react, the product includes more than one phase of an aqueous gel, generally described by the formula Ti. O 2 n. HH 2 O. 3. The product of this reaction appears as a yellowish gel after additional clearing procedures. Theodore Eliades, JOMI 1997

The effect of ROD on the implant material surface properties : hydrogen peroxide 4. It has been speculated that this specific interaction between the titanium and H 2 O 2 may account for the growth of the oxide layer, the biocompatibility of the implant, and the decreased rate of infection occurrence associated with implants. 5. The proposed mechanism for these effects includes the chemical potential of H 2 O 2 as the “driving force” for diffusion of the Ti ions through the oxide and the resultant oxide increase in thickness. Theodore Eliades, JOMI 1997

The effect of ROD on the implant material surface properties : O 2 and titanium oxides 1. Titanium forms several oxides (Ti. O 2, Ti. O, Ti 2 O 5), with Ti. O 2 being the most common. 2. Ti. O 2 is probably the most stable oxide, with three crystalline forms : the tetragonal anatase and rutile, and the orthorhombic brookite. Theodore Eliades, JOMI 1997

Passive film growth : experimental evidence 1. Auger and x-ray photoelectron spectroscopic studies have confirmed growth of the oxide layer, presenting proof of a continuous film-thickening pattern of growth occurring in an outward direction, while others have found that oxide-layer formation follows a different pattern of growth. 2. Spectroscopic analyses have also revealed that the thickened film is comprised mainly of Ca, P, and S, and therefore, Ti. O 2 is not the predominant substance. Theodore Eliades, JOMI 1997

Passive film growth : experimental evidence 3. The fact that P and S are present can be attributed to the “naturally” occurring initial precipitation of P on Ti surfaces, which allows the adsorbance of Ca, leading to the formation of calcium phosphate (Ca 3 [PO 4] 2) at ordinary conditions. 4. Studies have also proposed that oxide growth occurs inward from the bulk material and that a regulatory process through the dissociation from the hydroxylated surface of Ti(OH) and Ti-H-PO 4 takes place. Theodore Eliades, JOMI 1997

p. H of bioliquid and Calcium phosphate formation 1. It has been reported that trauma induced by the implant-placement procedure decreases the p. H of the bioliquid surrounding the implant site, resulting in a more acidic environment. 2. Decreased p. H, found to be consistent with increased [Ca]/[P] ratio, is indicative of the increased rate of calcium phosphate formation in vivo because of the acidic environment resulting from the low p. H value of the bioliquid. Theodore Eliades, JOMI 1997

p. H of bioliquid and Calcium phosphate formation 3. Therefore, it seems that Ti. O 2 offers the substrate for calcium-phosphate crystal formation, which may account for the proven biocompatibility of this oxide, since the tissue in contact with the implant “experiences” a layer of a bioinert substance. 4. The difference between the HA implants and the Ti. O 2, as far as biocompatibility is concerned, pertains to the fact that for the calcium phosphate to express its biologic properties, a layer of at least 1 m is required and therefore these properties are not readily available, as they are in the case of HA. Theodore Eliades, JOMI 1997

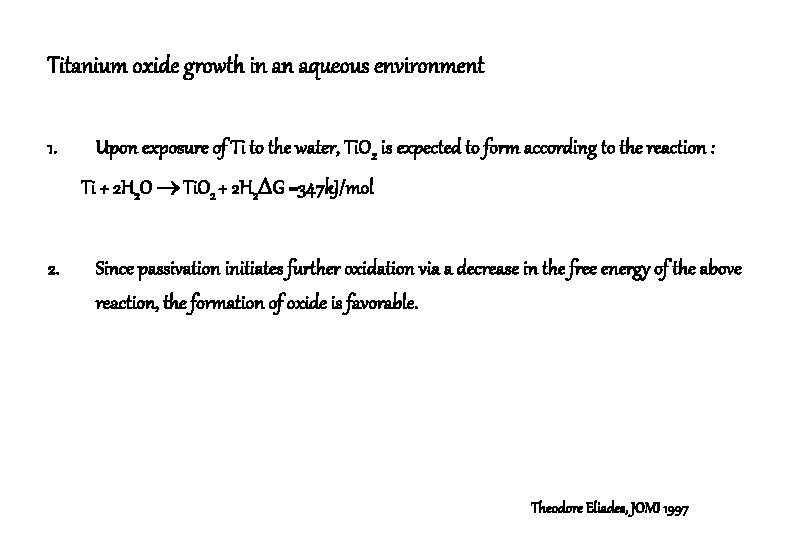
Titanium oxide growth in an aqueous environment 1. Upon exposure of Ti to the water, Ti. O 2 is expected to form according to the reaction : Ti + 2 H 2 O Ti. O 2 + 2 H 2 G =347 k. J/mol 2. Since passivation initiates further oxidation via a decrease in the free energy of the above reaction, the formation of oxide is favorable. Theodore Eliades, JOMI 1997

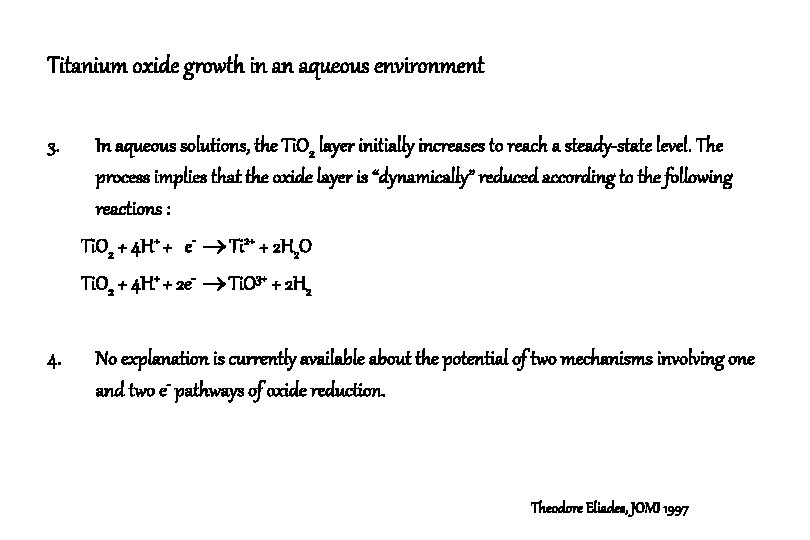
Titanium oxide growth in an aqueous environment 3. In aqueous solutions, the Ti. O 2 layer initially increases to reach a steady-state level. The process implies that the oxide layer is “dynamically” reduced according to the following reactions : Ti. O 2 + 4 H+ + e- Ti 2+ + 2 H 2 O Ti. O 2 + 4 H+ + 2 e- Ti. O 3+ + 2 H 2 4. No explanation is currently available about the potential of two mechanisms involving one and two e- pathways of oxide reduction. Theodore Eliades, JOMI 1997

Titanium oxide growth in an aqueous environment 4. The reason for attaining a constant-thickness value might be that ion migration through the crystalline layer requires higher electric fields than that of the amorphous layer. 5. Thus, a self-limiting process for oxide growth may be involved. Theodore Eliades, JOMI 1997

Biologic considerations of passive film growth 1. It is known that blood flow in marrow is approximately 20 to 25 times higher than in the cortex, and consequently, the PO 2 and (O 2) are expected to be similarly high, possibly accelerating oxide growth. 2. Results supporting this hypothesis, showing that the site of implantation affected the thickness of the oxide layer on implant materials, have been presented, demonstrating that in bone-cortex sites, oxide thickness was 3 to 4 times thinner than in bone marrow. Theodore Eliades, JOMI 1997

Biologic considerations of passive film growth 3. Metabolic activity may also influence oxide layer. During high-bond breakdown, which is induced by the increased metabolic activity of the tissue insult at the implant site, energy release occurs, which may affect the oxidation potential of the implant material. (phosphate bond in adenosine triphosphate provides 42 KJ/mol) Theodore Eliades, JOMI 1997

Biologic considerations of passive film growth 4. It is possible that film thickness may also be attributed to adsorption of blood proteins. 5. Although albumin has been found to inhibit the calcium phosphate formation in vitro in commercially pure titanium, increased passive film thickness has been described following exposure of H 2 O to this protein. Theodore Eliades, JOMI 1997

Conclusion 1. Growth of Ti. Ox may be explained through several processes derived from thermodynamic, electrochemical, and biologic approaches. 2. The models proposed to substantiate the phenomenon are often contradictory, suggesting passive film growth inward or outward from the bulk material, with increasing or selflimiting rate of oxide formation as a function of time. Theodore Eliades, JOMI 1997

Conclusion 3. Nevertheless, in vivo experimental observations are consistent with aging-induced thickening of the complexes precipitated on the implant surface. Theodore Eliades, JOMI 1997

Wear particles and surface topographies are modulations of osteoclastogenesis in vitro Beatrice Sommer, Rolf Felix, Christoph Sprecher, Michael Leunig, Reinhold Ganz, Willy Hofstetter J. Biomed. Mater. Res. 2005, 72 A: 67 -76

Success of orthopedic implants and their longevity Depends on 1. Primary stability 2. Bonding of the periimplant tissue to the implant 3. Development of chronic inflammations caused by the shedding of wear particles from the implant Sommer et al. , JBMR 2005

Implant surface characteristics and Wear particles 1. A structured surface may enable tighter bonding between implant and periprosthetic bone, but may also be prone to shed particles. 2. Polished surfaces may form a less tight implant-tissue interface, but may shed less particles. Sommer et al. , JBMR 2005

Wear particles and cell responses 1. The accumulation of wear particles in the periprosthetic tissue causes a chronic aseptic inflammation, including the invasion of immune cells and the formation of foreign body giant cells. 2. These cells release inflammatory cytokines that have the capacity to activate bone resorption such as interleukin (IL)-1 and -1 , IL-6, and tumor necrosis factor (TNF) Sommer et al. , JBMR 2005

TNF effect on the osteoclastogenesis 1. A functional p 55 receptor was required to maintain the TNF effect on the formation of osteoclasts. 2. Because TNF is released by cells of the monocyte/macrophage lineages upon exposure to particles, the crucial role of the cytokine in the loosening of orthopedic implants is further underlined. Sommer et al. , JBMR 2005

TNF effect on the osteoclastogenesis 3. TNF stimulates bone resorption not only by itself, but it modulates the process also in synergism with the receptor activator of NF- B ligand (RANKL), another member of the TNF family of growth factors. 4. Through synergistic actions, low concentrations of TNF , in the appropriate hematopoietic environment, may exert deleterious effects on local bone metabolism. Sommer et al. , JBMR 2005

Modulators of cell biology in peri-implant tissues 1. Wear particles shed from the implant 2. Topography, chemistry, and the size of the implant surface exert strong effects on the biology of the cells which get into contact with it as well. Sommer et al. , JBMR 2005

Surface characteristics, wear particles and cell responses 1. In the past, it has been shown that cells of different origin, such as cells of the osteoblast and monocyte/macrophage lineages, become activated when in contact with rough, topographically complex surfaces, as compared with smooth and polished surfaces. 2. However, the size of the particles determines whether they are phagocytosed, or whether they elicit a foreign body reaction. Sommer et al. , JBMR 2005

Surface characteristics, wear particles and cell responses 3. Monocytes phagocytosing small wear particles release inflammatory modulators generating a microenvironment favorable for the development of osteoclasts, whereas larger particles elicit foreign body granulomas that recruit osteoclast progenitor cells to the site of inflammation. 4. However, particles not only affects the cells of the monocyte/ macrophage lineages in dependence of their size, because also osteoblast lineage cells can ingest small-sized particles. Sommer et al. , JBMR 2005

Surface characteristics, wear particles and cell responses 5. As a consequence, the functions of osteoblasts are altered, eventually decreasing their boneforming capacity while inducing the release of osteoclastogenic cytokines. 6. Because osteoclast precursors require cells of the osteoblast lineage to generate a suitable microenvironment for their development, in these studies a coculture system of primary osteoblasts and bone marrow cells was used. Sommer et al. , JBMR 2005

Surface topography and cell function 1. Although the material of the metal substratum did not affect osteoclastogenesis, consistently higher numbers of osteoclasts were counted in cultures grown on sandblasted than in cultures grown on polished surfaces. 2. Cell function thus seems to be affected more profoundly by the topography, rather than by the chemical composition, of the surface. Sommer et al. , JBMR 2005

Surface topography and cell function 3. Activation of cells of osteoblast and macrophage lineages has been reported to depend on the topography of the implant surface, although the contributions of surface chemistry and topography to the modulation of cell behavior cannot always be clearly distinguished. 4. Bone marrow stromal cells were found to decrease proliferation and to increase differentiated functions such as the synthesis of alkaline phosphatase or the formation of nodules and mineral deposition in vitro when cultured on metal surfaces with increasing roughness. Sommer et al. , JBMR 2005

Surface topography and cell function 5. The macrophage cell line J 774 A. 1 was found to be stimulated to release bone morphogenetic protein-2 upon culture on sandblasted surfaces, which was not the case on polished surfaces, or on tissue culture plastic. 6. An increase in the differentiated functions of osteoblasts may well be associated with an increase in the capacity of these cells to support the formation of osteoclasts, as was observed in the present study. Sommer et al. , JBMR 2005

Monocyte/macrophage activation and osteolysis 1. The cells of the monocyte/macrophage lineages upon activation release as their main products the inflammatory cytokines IL-1 and TNF , both potent stimulators of bone resorption in vitro and in vivo. 2. Monocytes upon exposure to wear particles release increased levels of TNF. Sommer et al. , JBMR 2005

Monocyte/macrophage activation and osteolysis 3. Different cellular events take place in monocytes/ macrophages exposed to small (sub- m) or larger (>10 m) wear particles. 4. Small particles are phagocytosed by monocytes/ macrophages, inhibiting their differentiation to osteoclasts. 5. In addition, the simultaneous inhibitory effects on the development of osteoblasts further mediate antiosteoclastogenic effects. Sommer et al. , JBMR 2005

Monocyte/macrophage activation and osteolysis 6. Larger particles that cannot be phagocytosed, however, induce the development of foreign body granulomas that lead to a chronic inflammation, recruiting activated inflammatory cells and potential osteoclast progenitors to the respective periprosthetic site. Sommer et al. , JBMR 2005

Wear particle size and osteoclastogenesis (In vitro) 1. The smallest particles (cp Ti < 1 m) were most efficient in inhibiting osteoclastogenesis, followed by the slightly larger Al. Ox particles (<10 m) and then even larger cp Ti 325 and cp Ti 100 particles. 2. Whereas small particles were not efficient in suppressing the formation of osteoclasts, the release of TNF by bone marrow cells was less pronounced in the presence of small particles as compared with larger ones. Sommer et al. , JBMR 2005

Wear particle size and osteoclastogenesis (In vitro) 3. A possible explanation may be that phagocytosing monocyte/ macrophages release lower amounts of the cytokine than do macrophages that are encountering larger particles that they are not able to phagocytose. Sommer et al. , JBMR 2005

Conclusion 1. Chemistry of the metal surface is of little importance, rather it is the structure of the surface or the size of the particles that affects osteoclastogenesis. 2. Because surfaces exposed to body fluid or culture media become rapidly oxidized and will be coated with proteins. 3. Cells attaching to the metal surface, therefore, may not be in direct contact with the metal itself, but rather with the biofilm deposited on the surface. Sommer et al. , JBMR 2005

Conclusion 1. Within this study it was shown that wear particles shed from an implant or the topography of implant surfaces can induce changes in the local hematopoietic microenvironment regulating the recruitment and activation of osteoclasts. 2. These local conditions may be causing the formation of osteolytic lesions in the periprosthetic bone, contributing to the loosening of the implant, which will eventually make revision surgery a necessity. Sommer et al. , JBMR 2005

Does endotoxin contribute aseptic loosening of orthopedic implants ? Edward M. Greenfiled, Yanming Bi, Ashraf A. Ragab, Victor M. Goldberg, Jennifer L. Nalepka J. Biomed. Mater. Res. 2005, 72 B: 179 -185 Greenfiled et al. , JBMR 2005

Definition of aseptic loosening 1. Aseptic loosening refers to loosening in the absence of clinical or microbiological signs of infection and does not exclude a role for subclinical levels of bacteria. 2. Moreover, there is growing evidence that subclinical levels of bacteria colonize orthopedic implants in many patients with aseptic loosening 3. Aseptic loosening is often associated with periprosthetic osteolysis induced by the large number of wear particles generated from the implants. Greenfiled et al. , JBMR 2005

Endotoxin vs Lipopolysaccharides 1. Lipopolysaccharide (LPS) is the classical endotoxin produced by Gram (-) bacteria. 2. However, both Gram (-) and Gram (+) bacteria also produce other molecules, such as peptidoglycan, lipoteichoic acid, and teichoic acid, that exert endotoxin-like biological activities. 3. The terms “ endotoxin” will therefore be used in a global sense to refer to LPS as well as other bacterially derived molecules that have similar biological effects and “LPS” will be used to specifically refer to the classified endotoxin produced by Gram (-) bacteria. Greenfiled et al. , JBMR 2005

Cellular mechanisms of implant loosening 1. Periprosthetic osteolysis is primarily driven by increased osteoclast differentiation, which causes a 20 - to 30 -fold increase in the number of osteoclasts in both cell culture, and in the murine calvarial model of particle-induced osteolysis. 2. A similar increase in osteoclast number has been observed in patients with aseptic loosening. Kadoya et al. J. Orthop. Res. 1996 473 -482 Greenfiled et al. , JBMR 2005

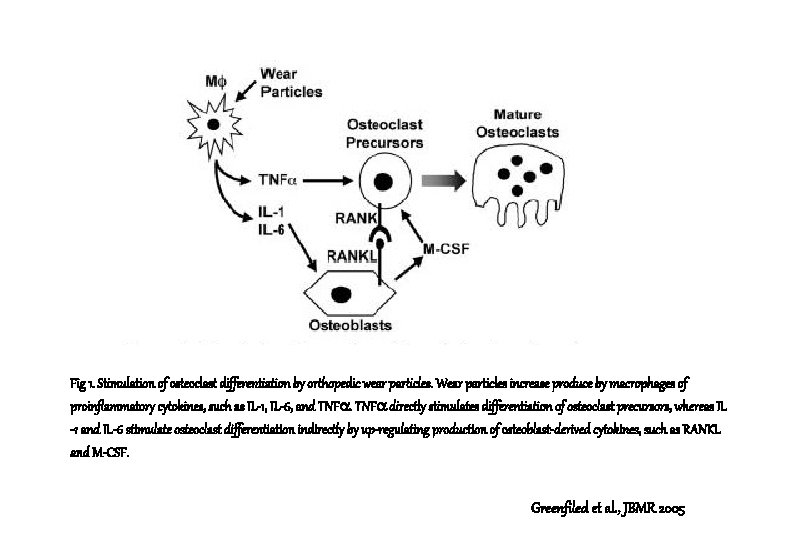
Fig 1. Stimulation of osteoclast differentiation by orthopedic wear particles. Wear particles increase produce by macrophages of proinflammatory cytokines, such as IL-1, IL-6, and TNF directly stimulates differentiation of osteoclast precursors, whereas IL -1 and IL-6 stimulate osteoclast differentiation indirectly by up-regulating production of osteoblast-derived cytokines, such as RANKL and M-CSF. Greenfiled et al. , JBMR 2005

Cellular mechanisms of implant loosening 3. Wear particles increase secretion of proinflammatory cytokines, including interleukine (IL) -1 , IL-6, and tumor necrosis factor (TNF) , by macrophages. 4. These cytokines synergistically stimulate differentiation of osteoclast precursors into mature, multinucleated osteoclasts that are capable of efficiently resorbing bone. Greenfiled et al. , JBMR 2005

Cellular mechanisms of implant loosening 5. TNF primarily acts directly on osteoclast precursors, whereas the remaining cytokines primarily act indirectly by increasing production, by osteoblasts, of cytokines such as RANKL and M-CSF, that, in turn, directly affect osteoclast precursors. Greenfiled et al. , JBMR 2005

Wear particles and Osteoclast differentiation 1. Osteoclast precursors are recruited both to wear particles implanted subcutaneously in mice and to pseudomembranes surrounding loose implants. 2. This recruitment of osteoclast precursors is likely due to increased production of chemotactic cytokines in response to the wear particles. Greenfiled et al. , JBMR 2005

Wear particles and Osteoclast & osteoblast activity 3. In contrast to the importance of precursor recruitment and their subsequent differentiation into mature osteoclasts, the contributions of increased osteoclast activity and survival appear to be relatively minor. 4. Similarly, with one exception, all in vitro studies on bone formation by osteoblasts have demonstrated very small effects of wear particles. In contrast, the inflammatory response induced by particles in vivo can substantially inhibit osseointegration. Greenfiled et al. , JBMR 2005

Wear particles and Osteoclast & osteoblast activity 5. The lack of major direct effects of particles on osteoblasts does not exclude a role for therapeutic approaches designed to stimulate bone formation because osteolysis is due to an imbalance between resorption and formation, 6. Active bone formation can repair particle-induced osteolysis in the murine calvarial model, and growth factor infusion can overcome the inhibition of osseointegration induced by particles. Greenfiled et al. , JBMR 2005

Polyethylene particles and osteolysis 1. We have recently shown that polyethylene particles induce osteolysis in the murine calvaria model by mechanisms indistinguishable from those responsible for osteolysis is induced by cp Ti particles. 2. It is therefore likely that our findings with cp Ti reflect general mechanisms by which wear particles induce osteolysis. 3. The possibility that endotoxin differentially adheres to particles of different material compositions or differentially affects their biological activity cannot be excluded at this time. Greenfiled et al. , JBMR 2005

Endotoxin and Increased biological activity of wear particles 1. Surprisingly at the time, the “endotoxin-free” cp. Ti particles were almost completely unable to stimulate cytokine production by murine marrow cells. 2. In contrast, the cp Ti particles with adherent endotoxin robustly stimulated production of both IL-6 and TNF. These observations have been extended to include measurement of IL- and osteoclast differentiation as well as to include incubation of the particles with human peripheral blood monocytes and with RAW 264. 7 murine macrophages. Greenfiled et al. , JBMR 2005

Endotoxin and Increased biological activity of wear particles 3. In all cases, cp Ti particles with adherent endotoxin induce significantly greater effects than do “endotoxin-free” particles. 4. In the experiment performed by Merkel et al. “endotoxin-free” cp Ti particles induce approximately 50% as much osteolysis as do cp Ti particles with adherent endotoxin. Greenfiled et al. , JBMR 2005

Endotoxin and Increased biological activity of wear particles 5. Endotoxin also accumulates similarly after implantation of endotoxin-free polyethylene particles. 6. In contrast, endotoxin is removed in vivo from cp Ti particles with adherent endotoxin. Taken together, these results emphasize the importance of the balance between accumulation and removal that leads to an equilibrium level of endotoxin. Greenfiled et al. , JBMR 2005

Endotoxin and Increased biological activity of wear particles 7. Interestingly, adherent endotoxin had no detectable effect on the rate or the extent of either attachment or internalization of cp Ti particles by RAW 264. 7 murine macrophages in culture conditions in which adherent endotoxin significantly increases both cytotoxin production and osteoclast differentiation induced by the particles. 8. Thus, adherent endotoxin increases the cellular responses to particles rather than their phagocytosis. Greenfiled et al. , JBMR 2005

Adherent endotoxin and osteoblast & macrophage response 1. Particles with adherent endotoxin induce significantly higher levels of signal transduction, cytokine production, osteoclast differentiation, and osteolysis. 2. In contrast, it has been reported that endotoxin-free particles exert similar effects on osteoblasts as do particles with endotoxin. 3. Thus, macrophage responses to wear particles may be much more affected by adherent endotoxin than are osteoblastic responses to particles. Greenfiled et al. , JBMR 2005

Implant loosening and related factors. 1. Implant motion, mechanical forces, or fluid pressure can increase the biological responses induced by wear particles. 2. Moreover, early implant migration is associated with a greater incidence of aseptic loosening leading some investigators to conclude that insufficient initial fixation is more important than particle generation in the development of loosening. Greenfiled et al. , JBMR 2005

Implant loosening and related factors. 3. However, because insufficient initial fixation also likely increases both particle generation and particle migration to the site of osteolysis. 4. It is also likely that implant motion is more important for inhibition of initial osseointegration, whereas particles are likely to be more important for subsequent induction of osteolysis. Greenfiled et al. , JBMR 2005

Implant loosening and related factors. 5. We therefore hypothesized that the endotoxin that is initially adherent to implants is more likely to affect early osseointegration rather than particle-induced osteolysis that occurs years after implantation Greenfiled et al. , JBMR 2005

3 possible potential sources of endotoxin 1. First, before surgery, the implants themselves may contain substantial amounts of adherent endotoxin. However, this endotoxin is restricted to the surface of the implants and can adhere to the initial particles that are generated from the surface but not to particles that are generated subsequently. 2. Second, the high affinity of wear particles for endotoxin may lead to accumulation of circulating endotoxin, derived from intestinal flora, minor infections, or dental procedures on the wear particles after they are generated in patients. Greenfiled et al. , JBMR 2005

3 possible potential sources of endotoxin 3. Third, it is likely that the quantitatively most important source of endotoxin is the subclinical bacterial biofilm found on many implants from patients with aseptic loosening. Greenfiled et al. , JBMR 2005

Bacterial biofilms and aseptic loosening 1. Many microbiological studies have documented the presence of bacterial biofilms, primarily Gram (+), on the surface of many orthopedic implants in the presence of any signs of clinical infection. 2. The potential importance of bacteria in aseptic loosening is emphasized by a Norwegian study of > 20, 000 total hip replacements showing that inclusion of antibiotics in the PMMA cement used for fixation of the implant reduces the frequency of aseptic loosening. Greenfiled et al. , JBMR 2005

3 possible potential sources of endotoxin 1. First, before surgery, the implants themselves may contain substantial amounts of adherent endotoxin. However, this endotoxin is restricted to the surface of the implants and can adhere to the initial particles that are generated from the surface but not to particles that are generated subsequently. 2. Second, the high affinity of wear particles for endotoxin may lead to accumulation of circulating endotoxin, derived from intestinal flora, minor infections, or dental procedures on the wear particles after they are generated in patients. Greenfiled et al. , JBMR 2005