Parts of a Solution n SOLUTE the part








- Slides: 16

Parts of a Solution n SOLUTE – the part of a solution that is being dissolved (usually the lesser amount) SOLVENT – the part of a solution that dissolves the solute (usually the greater amount) Solute + Solvent = Solution Solute Solvent Example solid Metal alloys solid liquid Salt water gas solid Moth balls liquid Alcohol in water gas liquid soda gas air

Solubility is • the maximum amount of solute that dissolves in a specific amount of solvent. • expressed as grams of solute in 100 grams of solvent water. g of solute 100 g water https: //www. youtube. com/watch? v=RJqts. C 8 Q-t. M 2

Factors That Affect Solubility and Rate of Dissolving

Factors That Affect Rate of Dissolving 1) Temperature, more specifically. . . as T ↑, rate of dissolving ↑. 2) Agitation Shaking, stirring 3) Surface area of solids. Smaller particles dissolve more quickly than larger ones. 4) Pressure (only for gasses) https: //www. youtube. com/watch? v=q. L 5 -lcc_Tf. Y

How would you use this information to prepare orange juice from the frozen concentrate as expeditiously as possible? defrost (liquefy) the concentrate(increase temperature) n shake or stir when water added (agitation) n

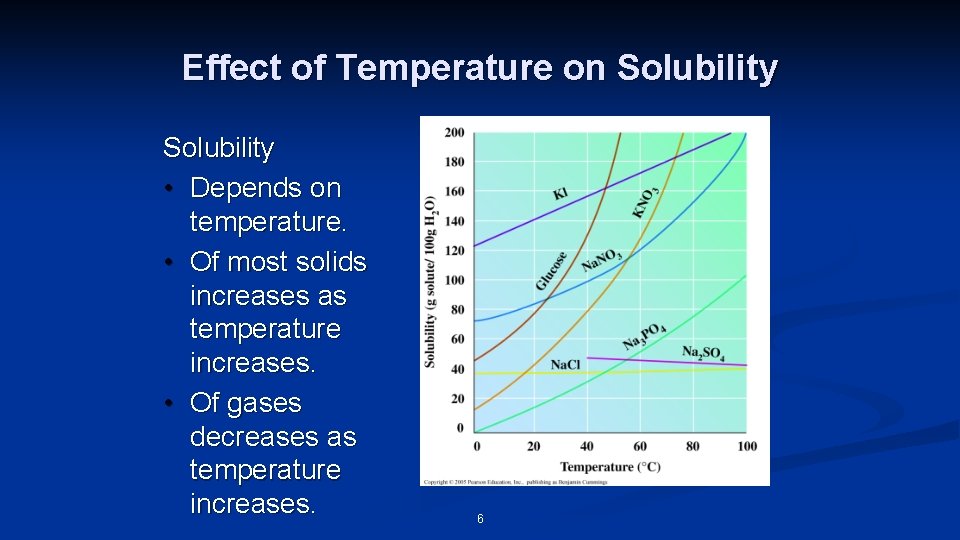
Effect of Temperature on Solubility • Depends on temperature. • Of most solids increases as temperature increases. • Of gases decreases as temperature increases. 6

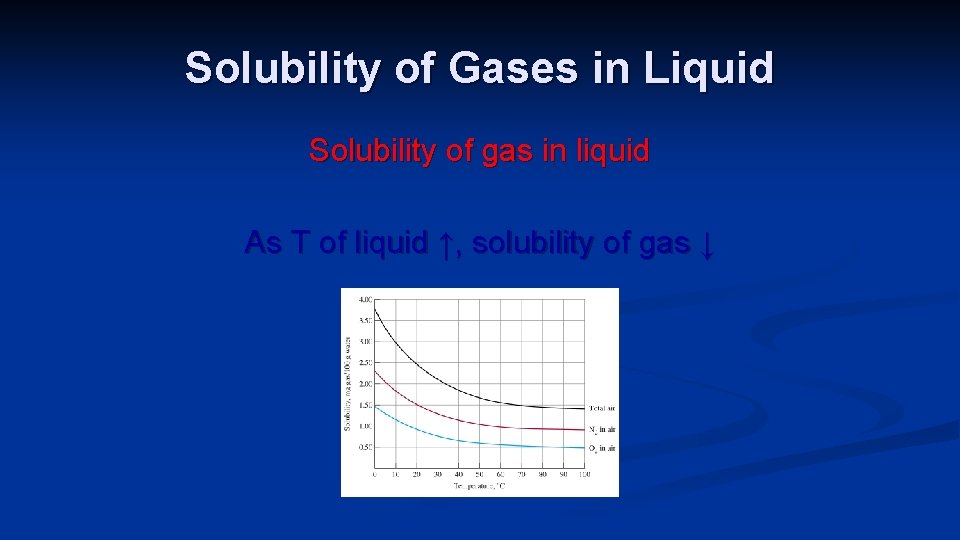
Solubility of Gases in Liquid Solubility of gas in liquid As T of liquid ↑, solubility of gas ↓

Heat Pollution of Water Many industries return warm water to a river or lake. What effect would this have? Dissolved O 2 ↓; marine life can suffer.

At which temperature will you get a fizzier glass of pop—cold or warm? Cold!! Why? Solubility of CO 2 ↓ as T ↑

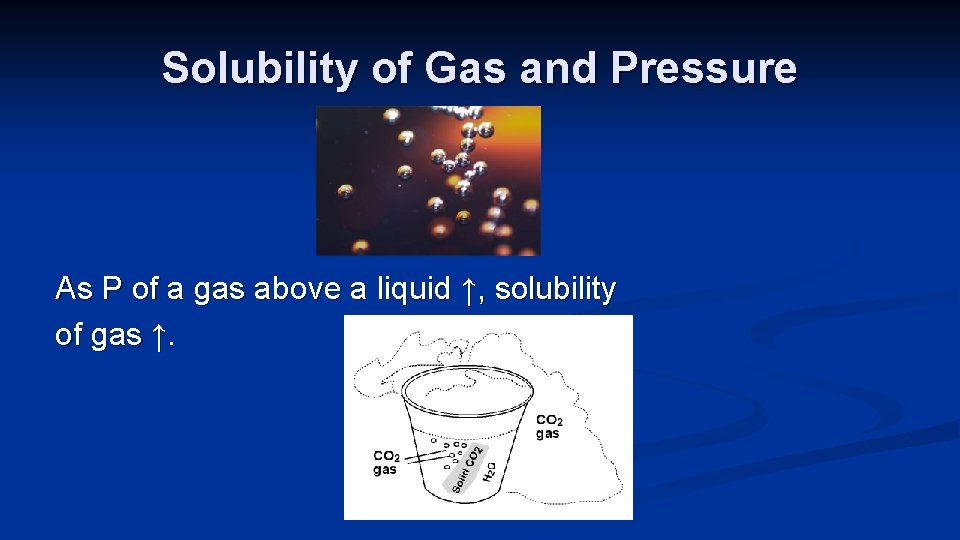
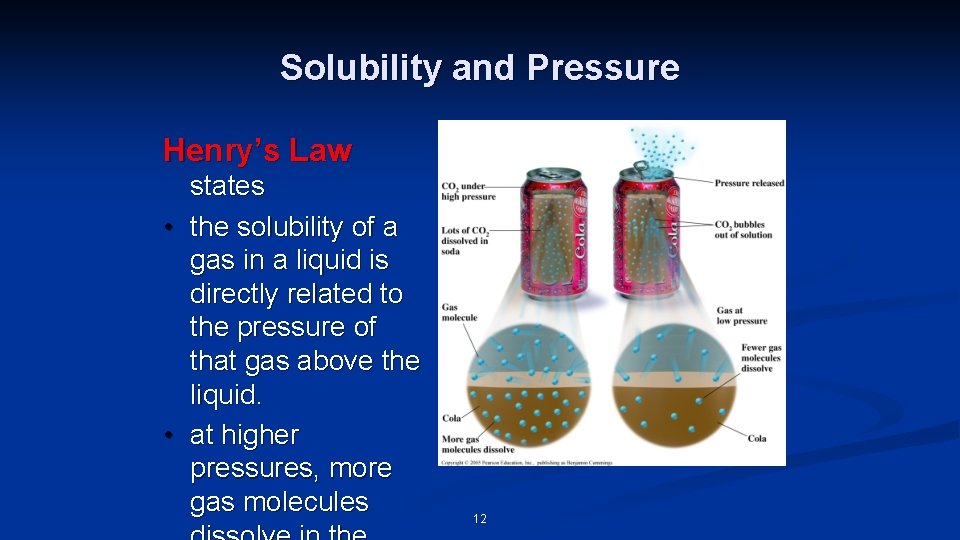
Solubility of Gas and Pressure As P of a gas above a liquid ↑, solubility of gas ↑.

A potential problem for SCUBA divers is narcosis, aka “the bends. ” N 2 dissolves in blood at high P under surface of water. If diver ascends too fast, N 2 bubbles out of the blood, much like CO 2 in pop. Can be painful—sometimes fatal.

Solubility and Pressure Henry’s Law • • states the solubility of a gas in a liquid is directly related to the pressure of that gas above the liquid. at higher pressures, more gas molecules 12

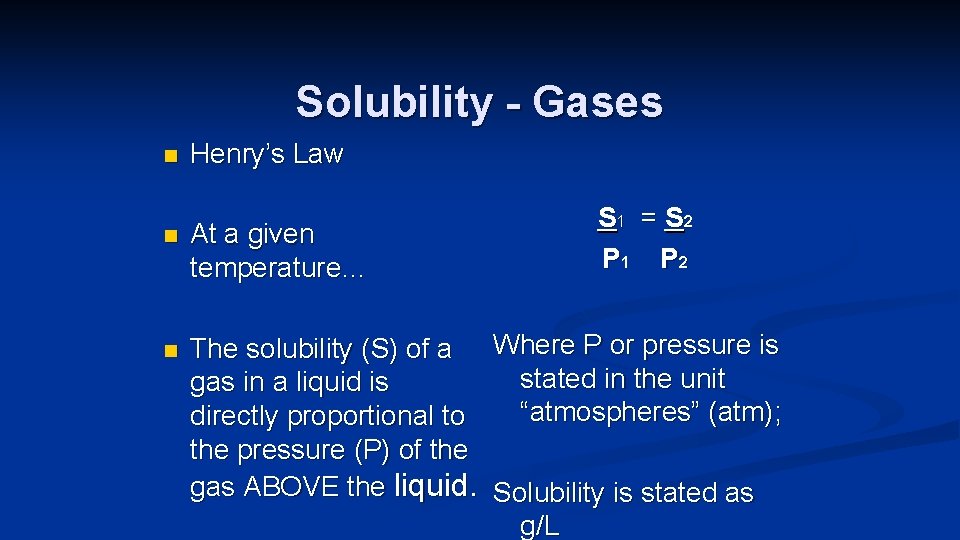
Solubility - Gases n n n Henry’s Law At a given temperature… S 1 = S 2 P 1 P 2 The solubility (S) of a Where P or pressure is stated in the unit gas in a liquid is “atmospheres” (atm); directly proportional to the pressure (P) of the gas ABOVE the liquid. Solubility is stated as g/L

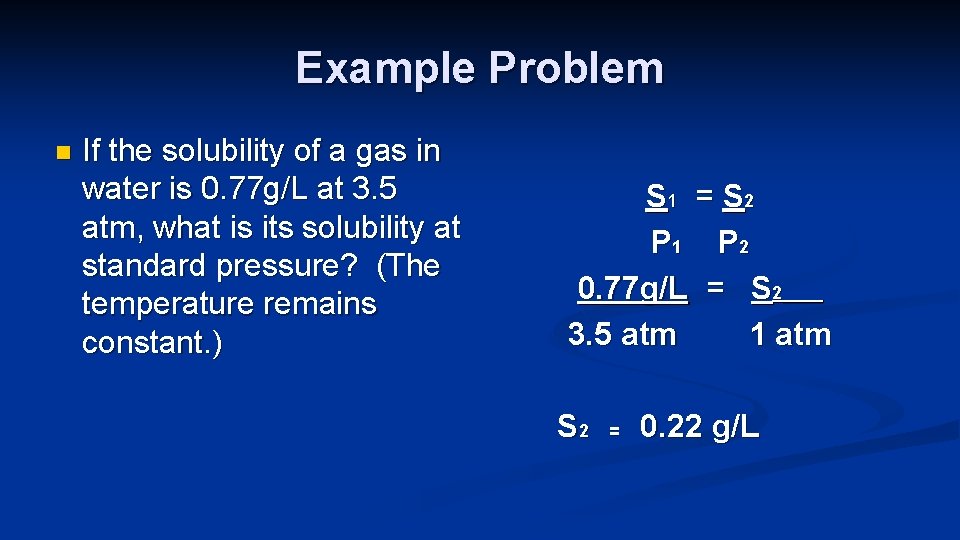
Example Problem n If the solubility of a gas in water is 0. 77 g/L at 3. 5 atm, what is its solubility at standard pressure? (The temperature remains constant. ) S 1 = S 2 P 1 P 2 0. 77 g/L = S 2____ 3. 5 atm 1 atm S 2 = 0. 22 g/L

What simple rule governs solubilty? Like dissolves like n Polar dissolves polar (and ionic). eg. CH 3 OH (methanol) dissolves in H 2 O. Na. Cl dissolves in water. n

Name something polar that doesn’t dissolve in water. A polar bear.