Particulate Model of Matter 22 1 What Matter










- Slides: 20

Particulate Model of Matter

22. 1 What Matter is Made Up of • What is matter made up of? • Ancient Greek philosophers thought that matter was made up of fire, air, earth and water. • However, scientists today have deduced from experiments that matter is made up of tiny particles that are in constant and random motion.

22. 2 Evidence for Moving Particles • The spreading of smells, such as from cooking or perfumes, is because of the movement of tiny particles in air. • These particles move about randomly in all directions, and hence the smells spread. • We say that diffusion has taken place. • Diffusion is a process by which particles of matter move from a region of higher concentration to a region of lower concentration.

22. 2 Evidence for Moving Particles Experiment 1: Diffusion in Gases Prepare a gas jar of bromine vapour by placing a few drops of liquid bromine in the gas jar. Then invert a gas jar containing air over it. Observe what happens. After some time, both gas jars become filled uniformly with a reddish-brown colouration.

22. 2 Evidence for Moving Particles • Diffusion also occurs in liquids. • However, it takes place more slowly than in gases. • This shows that particles in a liquid move around more slowly than in gases.

22. 2 Evidence for Moving Particles Experiment 2: Diffusion in Liquids Place a few crystals of potassium permanganate at the bottom of a beaker of water. Leave the set-up to stand observe it from time to time. water potassium permanganate crystals At the beginning After a few days, the purple colouration of potassium permanganate will spread throughout the mixture.

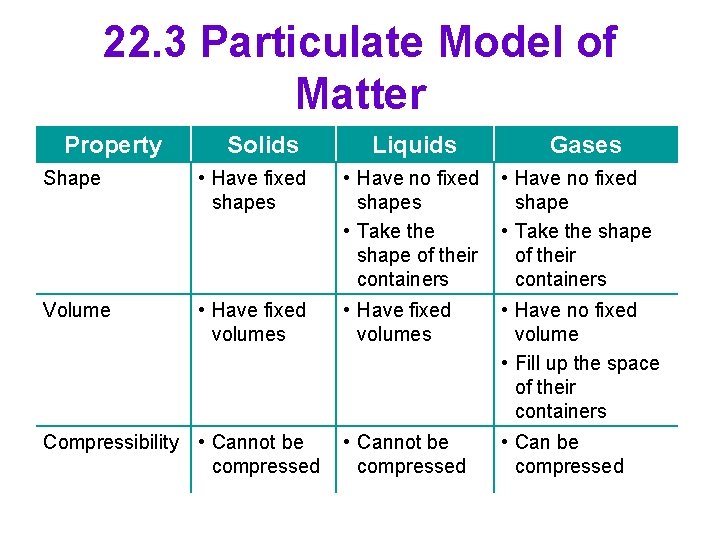
22. 3 Particulate Model of Matter Property Solids Liquids Gases Shape • Have fixed shapes • Have no fixed shapes • Take the shape of their containers • Have no fixed shape • Take the shape of their containers Volume • Have fixed volumes • Have no fixed volume • Fill up the space of their containers • Cannot be compressed • Can be compressed Compressibility • Cannot be compressed

22. 3 Particulate Model of Matter • Scientists use the particulate model of matter to explain that the three states of matter are different because of the differences in the movement (motion) and arrangement of the particles. • We can use the particulate model to explain why the behaviour of solids, liquids and gases differs form one another.

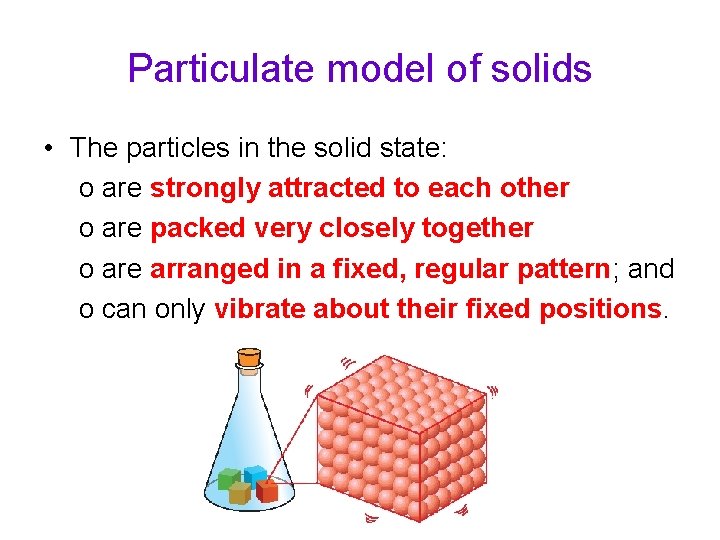
Particulate model of solids • The particles in the solid state: o are strongly attracted to each other o are packed very closely together o are arranged in a fixed, regular pattern; and o can only vibrate about their fixed positions.

Particulate model of solids • The particles in a solid are unable to move freely. Thus, a solid has a definite shape and volume. • There is no space between the particles to enable them to get any closer to one another. This is why solids cannot be compressed.

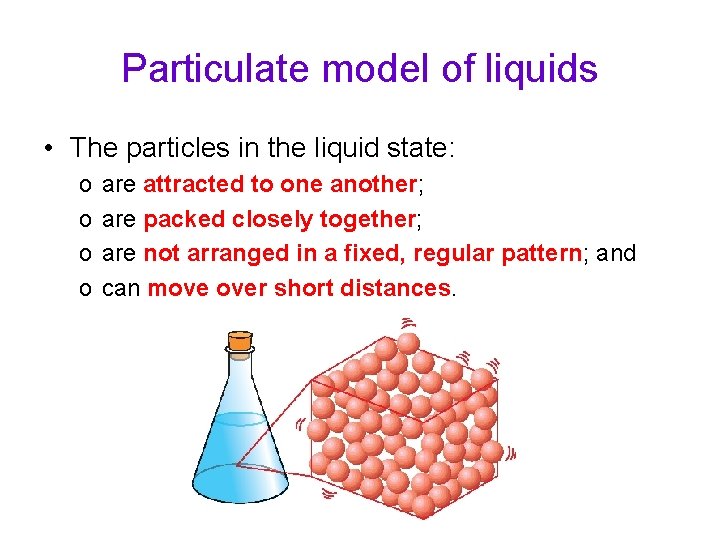
Particulate model of liquids • The particles in the liquid state: o o are attracted to one another; are packed closely together; are not arranged in a fixed, regular pattern; and can move over short distances.

Particulate model of liquids • The particles in the liquid state are farther away from one another than the particles in a solid. • However, the particles in a liquid are still held closely together. • Thus, like solids, liquids have a fixed volume and cannot be compressed. • Unlike solids, the particles in a liquid are not fixed in regular positions and are able to slide past one another. This is why a liquid has no definite shape.

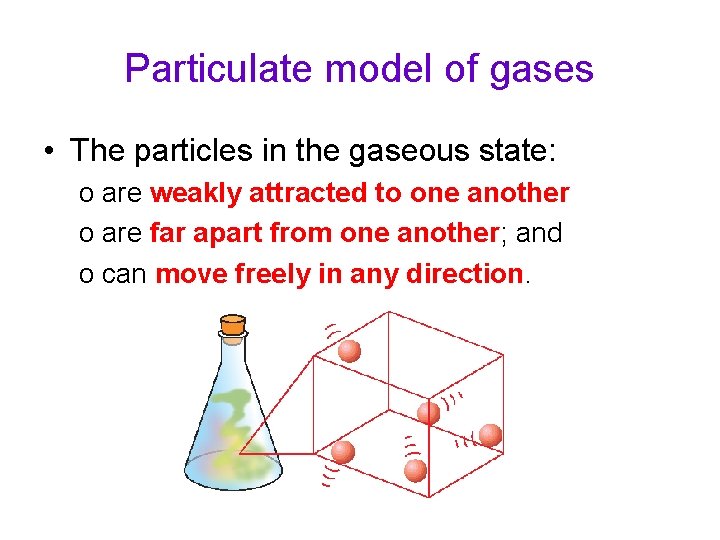
Particulate model of gases • The particles in the gaseous state: o are weakly attracted to one another o are far apart from one another; and o can move freely in any direction.

Particulate model of gases • The particles in a gas are spaced far apart from one another and so a gas can be compressed easily. • The particles are also able to move freely to occupy any available space. • This explains why a gas has no definite shape or volume.

22. 4 Changes of States of Matter • Matter can exist in the solid, liquid or gaseous state, depending on its temperature and atmospheric pressure. • At a fixed pressure, the temperature of an object will determine its state. Boiling occurs when ice is heated to a temperature of 0 0 C and above. Boiling occurs when liquid water is heated to a temperature of 100 0 C. Freezing occurs when water is cooled to 0 0 C and below. Condensation occurs when water vapour is cooled to a temperature of 100 0 C and below.

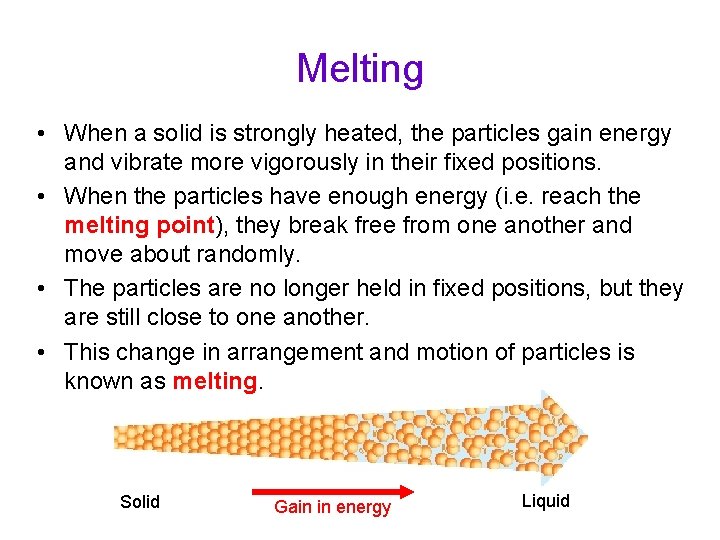
Melting • When a solid is strongly heated, the particles gain energy and vibrate more vigorously in their fixed positions. • When the particles have enough energy (i. e. reach the melting point), they break free from one another and move about randomly. • The particles are no longer held in fixed positions, but they are still close to one another. • This change in arrangement and motion of particles is known as melting. Solid Gain in energy Liquid

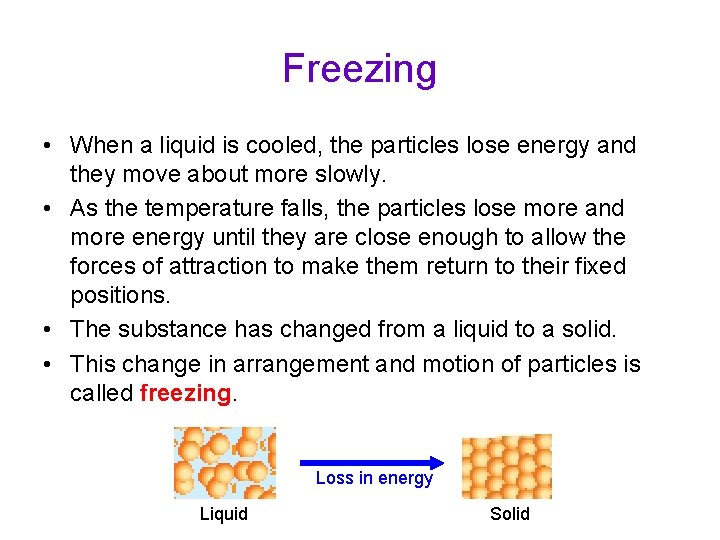
Freezing • When a liquid is cooled, the particles lose energy and they move about more slowly. • As the temperature falls, the particles lose more and more energy until they are close enough to allow the forces of attraction to make them return to their fixed positions. • The substance has changed from a liquid to a solid. • This change in arrangement and motion of particles is called freezing. Loss in energy Liquid Solid

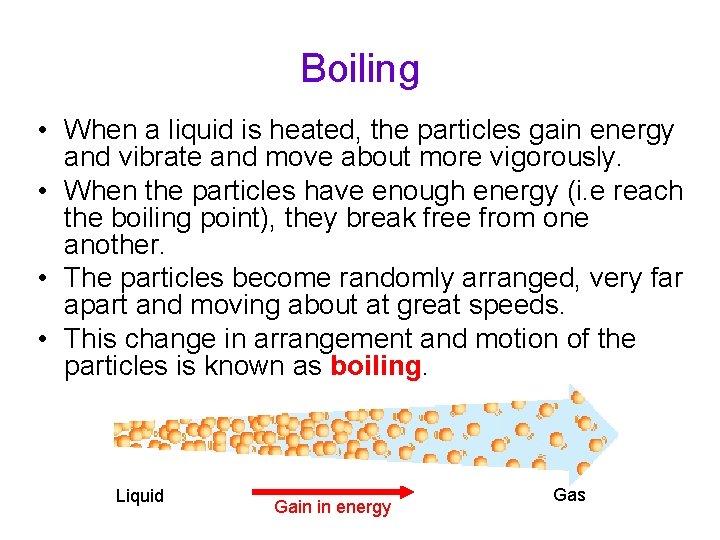
Boiling • When a liquid is heated, the particles gain energy and vibrate and move about more vigorously. • When the particles have enough energy (i. e reach the boiling point), they break free from one another. • The particles become randomly arranged, very far apart and moving about at great speeds. • This change in arrangement and motion of the particles is known as boiling. Liquid Gain in energy Gas

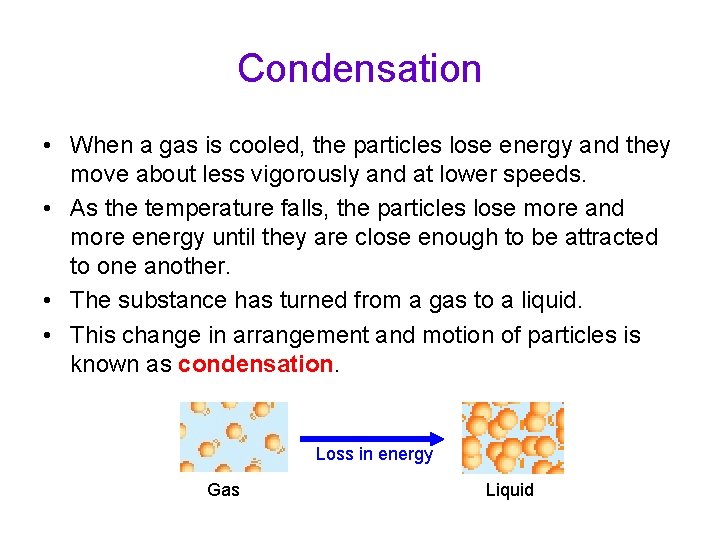
Condensation • When a gas is cooled, the particles lose energy and they move about less vigorously and at lower speeds. • As the temperature falls, the particles lose more and more energy until they are close enough to be attracted to one another. • The substance has turned from a gas to a liquid. • This change in arrangement and motion of particles is known as condensation. Loss in energy Gas Liquid

Miscellaneous • http: //www. bbc. co. uk/schools/ks 3 bitesize/s cience/chemistry/particle_model_intro. sht ml • http: //www. chem 4 kids. com/files/matter_int ro. html • http: //www. abpischools. org. uk/resources/s olids-liquids-gases/slg 2. asp • http: //www. chem. purdue. edu/gchelp/liquid s/character. html