Particles of Matter Chapter 1 3 Atomic Theory







- Slides: 9

Particles of Matter Chapter 1 -3

Atomic Theory �Atomic theory can be traced back to Democritus (460 -370 BC) who believed that matter could be divided into indivisible particles “atoms. ” The atomist theory was squashed by Aristotle and Plato and remained unpopular for almost 2000 years!

Atomic Theory �Newton, Galileo, and other luminaries embraced the idea of atoms in explaining phenomena like wind, but failed to see atoms as chemical building blocks.

John Dalton �John Dalton devised a chemical atomic theory between 1803 and 1807. While his postulates would be proven incorrect in the coming hundred years, the groundwork for an atomic theory of matter was set and the chemical age was born! !

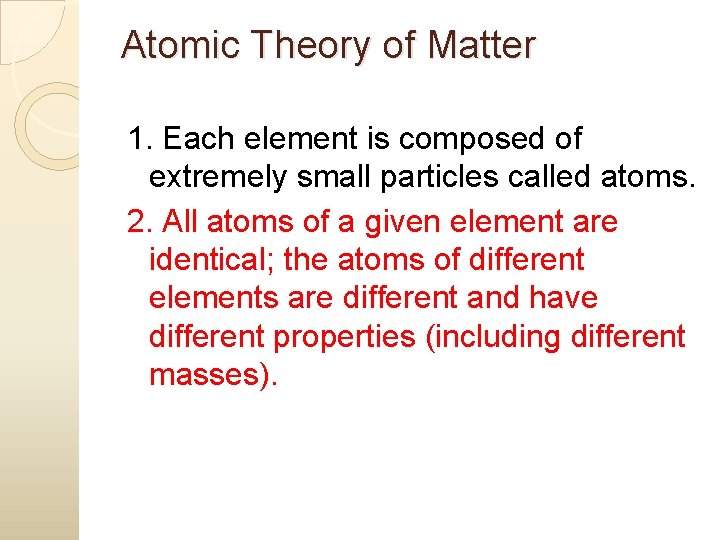
Atomic Theory of Matter � 1. Each element is composed of extremely small particles called atoms.

Atomic Theory of Matter 1. Each element is composed of extremely small particles called atoms. 2. All atoms of a given element are identical; the atoms of different elements are different and have different properties (including different masses).

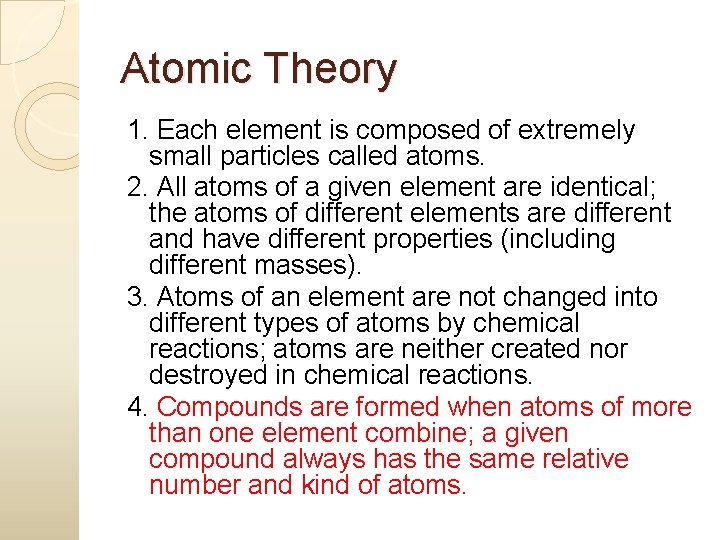
Atomic Theory 1. Each element is composed of extremely small particles called atoms. 2. All atoms of a given element are identical; the atoms of different elements are different and have different properties (including different masses). 3. Atoms of an element are not changed into different types of atoms by chemical reactions; atoms are neither created nor destroyed in chemical reactions.

Atomic Theory 1. Each element is composed of extremely small particles called atoms. 2. All atoms of a given element are identical; the atoms of different elements are different and have different properties (including different masses). 3. Atoms of an element are not changed into different types of atoms by chemical reactions; atoms are neither created nor destroyed in chemical reactions. 4. Compounds are formed when atoms of more than one element combine; a given compound always has the same relative number and kind of atoms.

Constant Ratio of Compounds �Water, no matter where it is collected, is chemically identical. It is a compound. �By contrast, sea water does not have a unique composition. It is a mixture of water, salt, potassium chloride, and many other compounds in smaller amounts.