Particles of Matter Atoms and Molecules The smallest



- Slides: 20

Particles of Matter

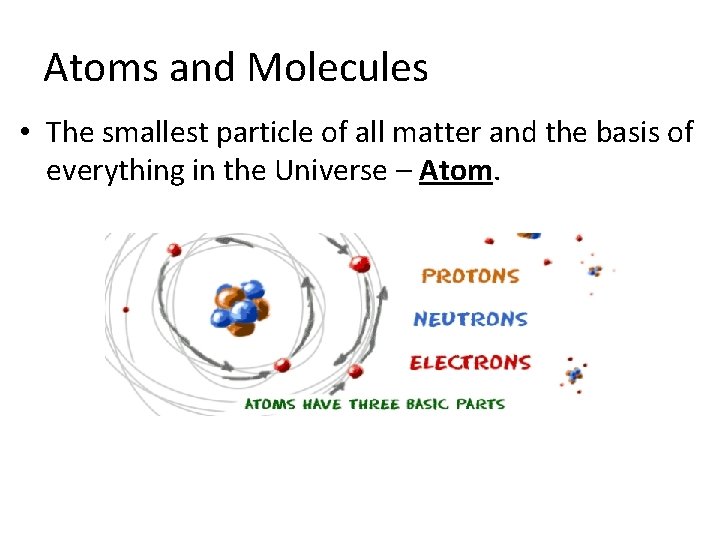
Atoms and Molecules • The smallest particle of all matter and the basis of everything in the Universe – Atom.

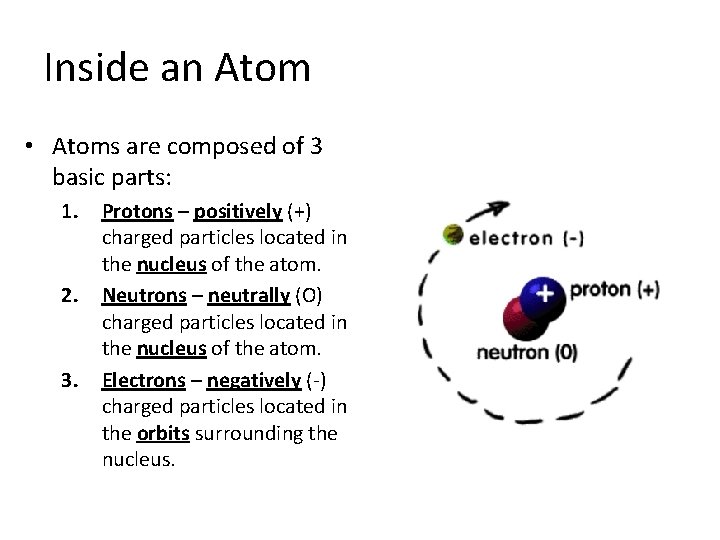
Inside an Atom • Atoms are composed of 3 basic parts: 1. 2. 3. Protons – positively (+) charged particles located in the nucleus of the atom. Neutrons – neutrally (O) charged particles located in the nucleus of the atom. Electrons – negatively (-) charged particles located in the orbits surrounding the nucleus.

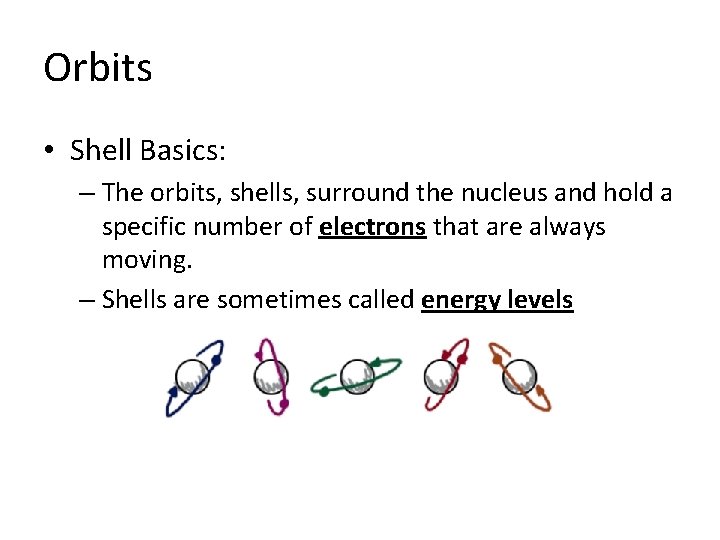
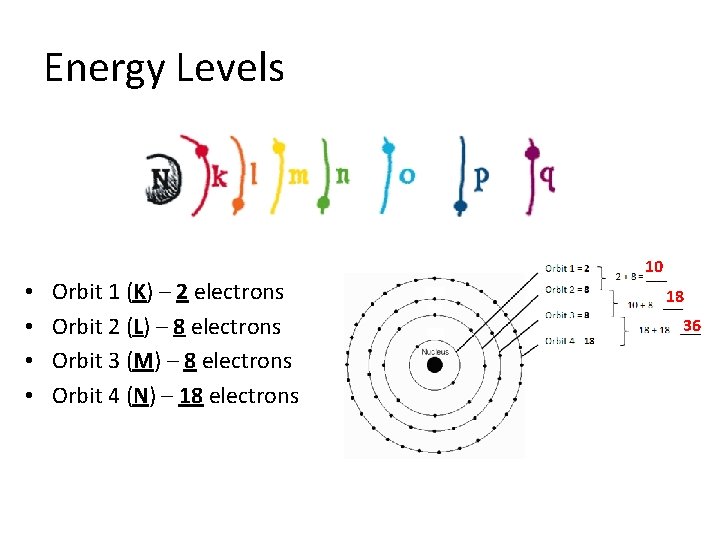
Orbits • Shell Basics: – The orbits, shells, surround the nucleus and hold a specific number of electrons that are always moving. – Shells are sometimes called energy levels

Energy Levels • • Orbit 1 (K) – 2 electrons Orbit 2 (L) – 8 electrons Orbit 3 (M) – 8 electrons Orbit 4 (N) – 18 electrons 10 18 36

Scientists Behind Atoms 1. Democritus • Greek philosopher • Lived about 440 B. C. • He thought that there were smallest possible “pieces” of everything. – Called this smallest piece, atomos – Greek for “uncuttable”.

Scientists Behind Atoms 2. Dalton • British schoolteacher – 1802 • Proposed the atomic theory – Theory about atoms • Dalton’s ideas form the basis of our understanding of atoms!

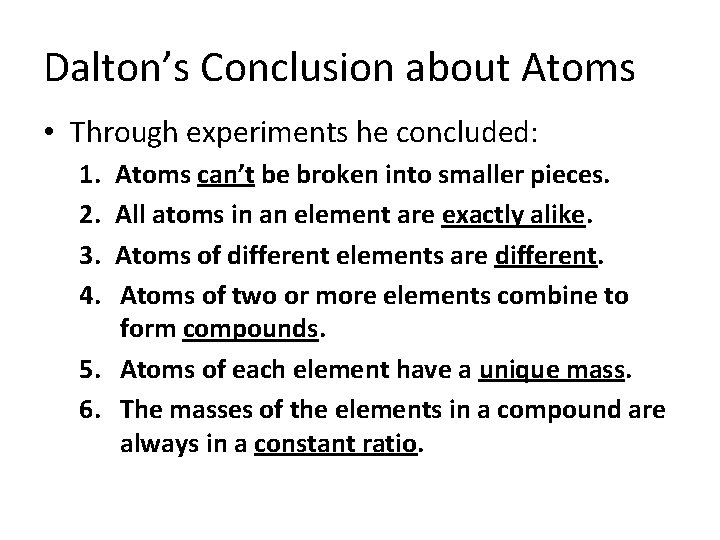
Dalton’s Conclusion about Atoms • Through experiments he concluded: 1. 2. 3. 4. Atoms can’t be broken into smaller pieces. All atoms in an element are exactly alike. Atoms of different elements are different. Atoms of two or more elements combine to form compounds. 5. Atoms of each element have a unique mass. 6. The masses of the elements in a compound are always in a constant ratio.

Organizing Elements 3. Dmitri Mendeleev – 1800’s, Russian scientist – Thought elements could be organized by: A. Similar chemical and physical properties B. Atomic mass C. Bonding power

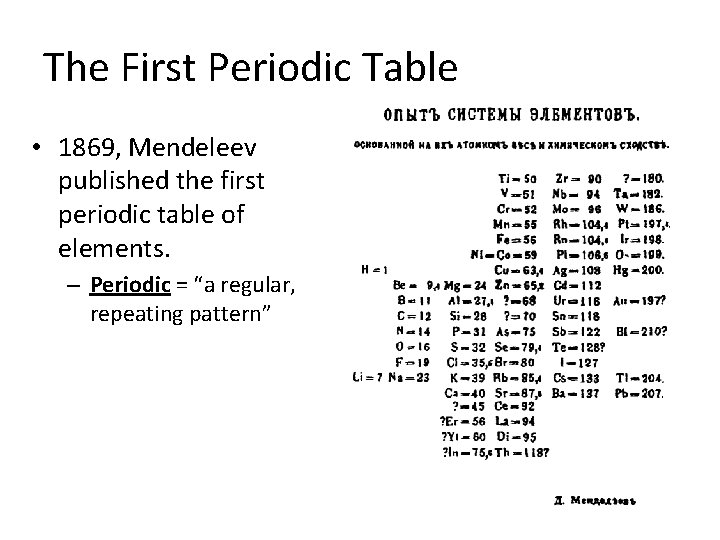
The First Periodic Table • 1869, Mendeleev published the first periodic table of elements. – Periodic = “a regular, repeating pattern”

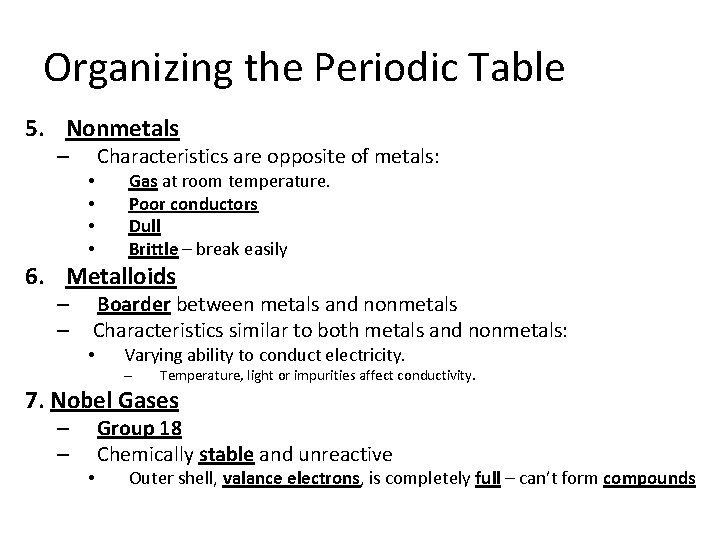
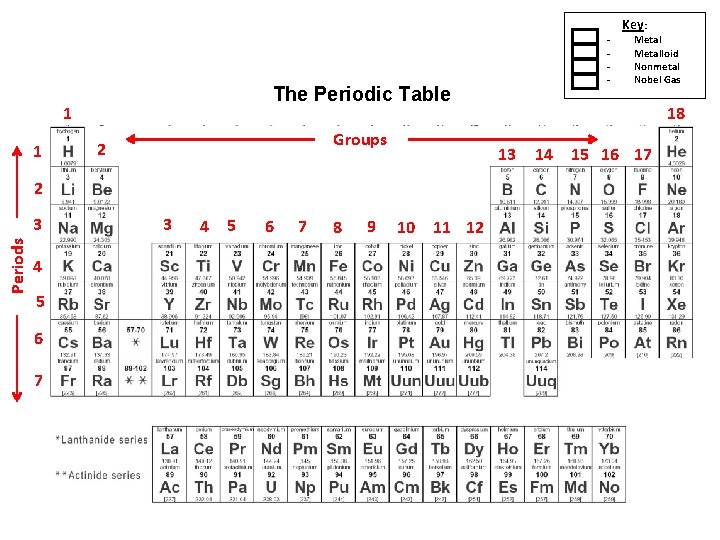
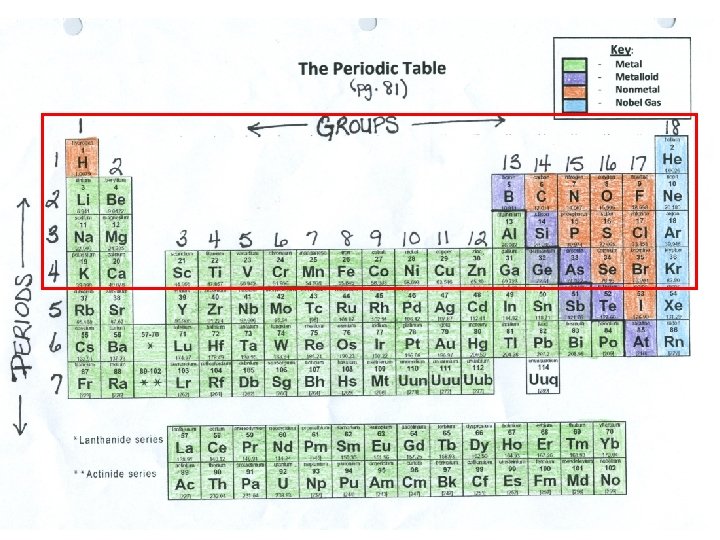
Organizing the Periodic Table 1. Arranged by atomic number 2. Groups (families): – Vertical columns down, 1 -18. – Based on similar characteristics 3. Periods : horizontal rows across, 1 -7. – 4. Metals – • • • Classified based on physical properties such as: Hardness Shininess Malleability – can be pounded into shape Ductility – can be pulled or drawn into a long wire Good conductors – transmit heat and electricity easily. Solid at room temperature.

Organizing the Periodic Table 5. Nonmetals – • • Characteristics are opposite of metals: Gas at room temperature. Poor conductors Dull Brittle – break easily 6. Metalloids – – Boarder between metals and nonmetals Characteristics similar to both metals and nonmetals: • Varying ability to conduct electricity. – Temperature, light or impurities affect conductivity. 7. Nobel Gases – – • Group 18 Chemically stable and unreactive Outer shell, valance electrons, is completely full – can’t form compounds

The Periodic Table 1 1 Groups 2 Periods 4 5 6 7 3 4 5 6 7 8 9 10 11 12 Key: Metalloid Nonmetal Nobel Gas 18 13 2 3 - 14 15 16 17


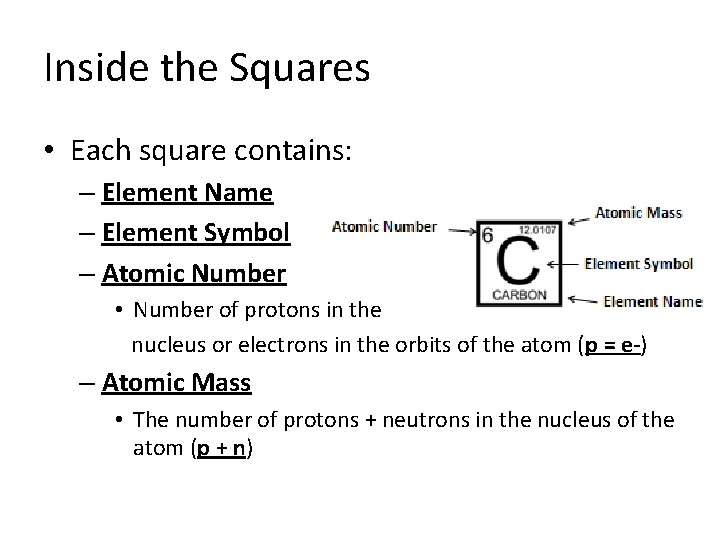
Inside the Squares • Each square contains: – Element Name – Element Symbol – Atomic Number • Number of protons in the nucleus or electrons in the orbits of the atom (p = e-) – Atomic Mass • The number of protons + neutrons in the nucleus of the atom (p + n)

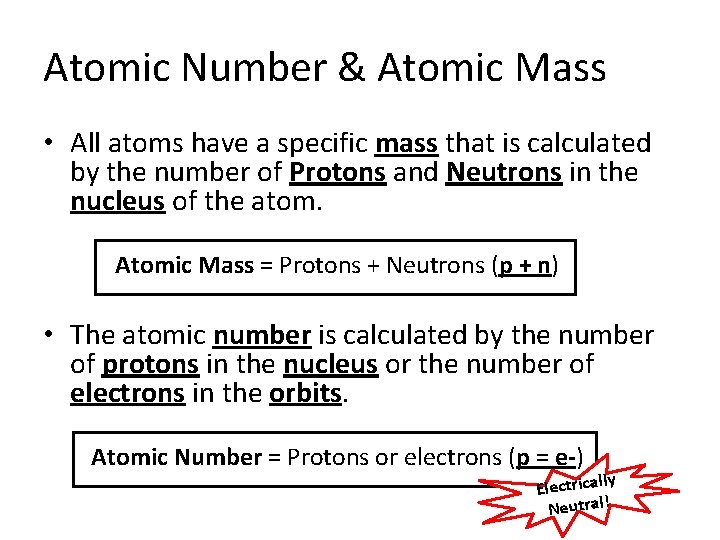
Atomic Number & Atomic Mass • All atoms have a specific mass that is calculated by the number of Protons and Neutrons in the nucleus of the atom. Atomic Mass = Protons + Neutrons (p + n) • The atomic number is calculated by the number of protons in the nucleus or the number of electrons in the orbits. Atomic Number = Protons or electrons (p = e-) ly Electrical Neutral!

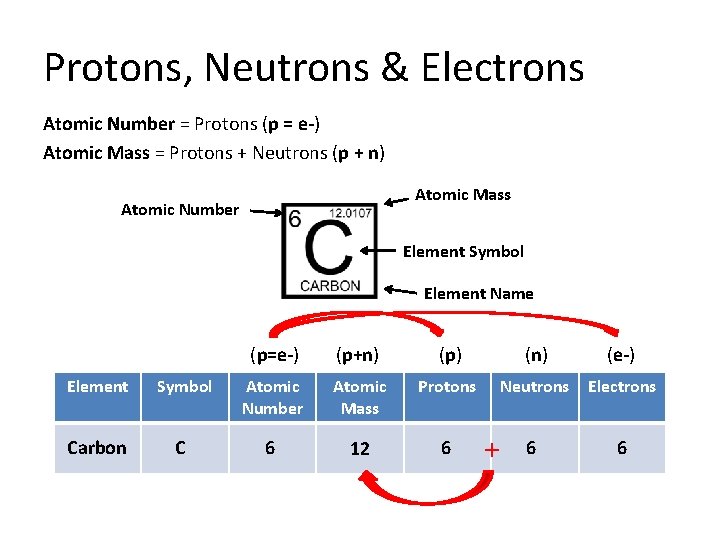
Protons, Neutrons & Electrons Atomic Number = Protons (p = e-) Atomic Mass = Protons + Neutrons (p + n) Atomic Mass Atomic Number Element Symbol Element Name (p=e-) (p+n) (p) (n) (e-) Neutrons Electrons Element Symbol Atomic Number Atomic Mass Protons Carbon C 6 12 6 + 6 6

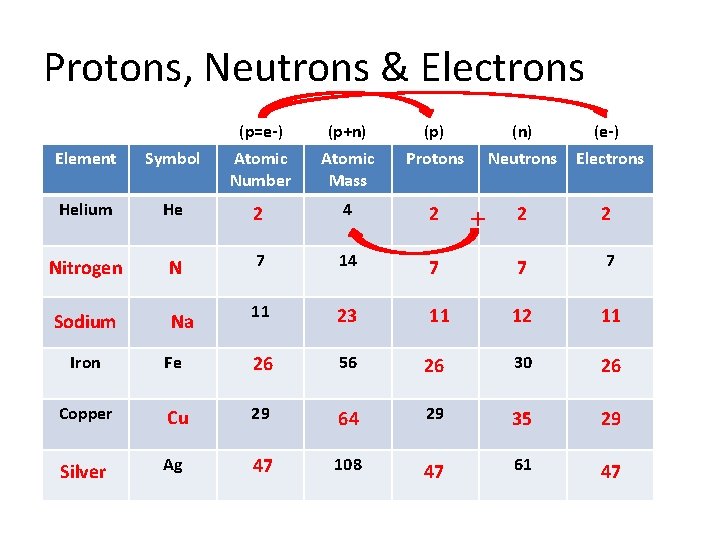
Protons, Neutrons & Electrons (p=e-) (p+n) (p) (n) (e-) Neutrons Electrons 2 2 Element Symbol Atomic Number Atomic Mass Protons Helium He 2 4 2 Nitrogen N 7 14 7 7 7 Sodium Na 11 23 11 12 11 + Iron Fe 26 56 26 30 26 Copper Cu 29 64 29 35 29 Silver Ag 47 108 47 61 47

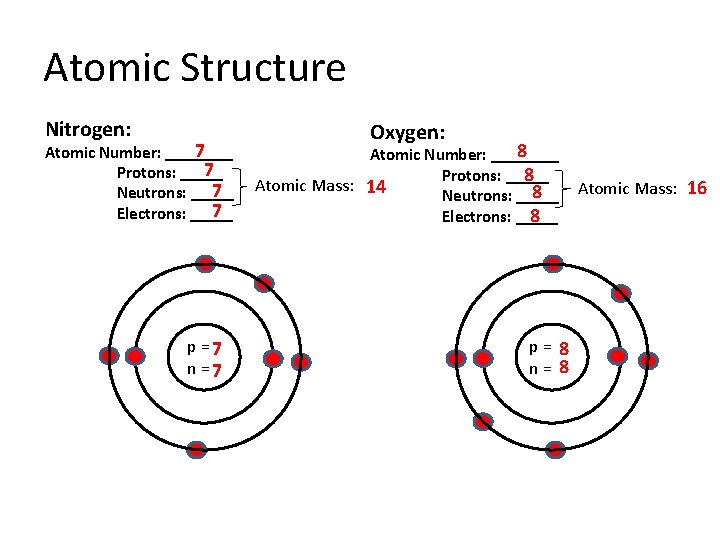
Atomic Structure Nitrogen: 7 Atomic Number: ____ 7 Protons: _____ 7 Neutrons: _____ 7 Electrons: _____ p =7 n =7 Oxygen: 8 Atomic Number: ____ Protons: _____ 8 Atomic Mass: 14 8 Neutrons: _____ Electrons: _____ 8 p= 8 n= 8 Atomic Mass: 16

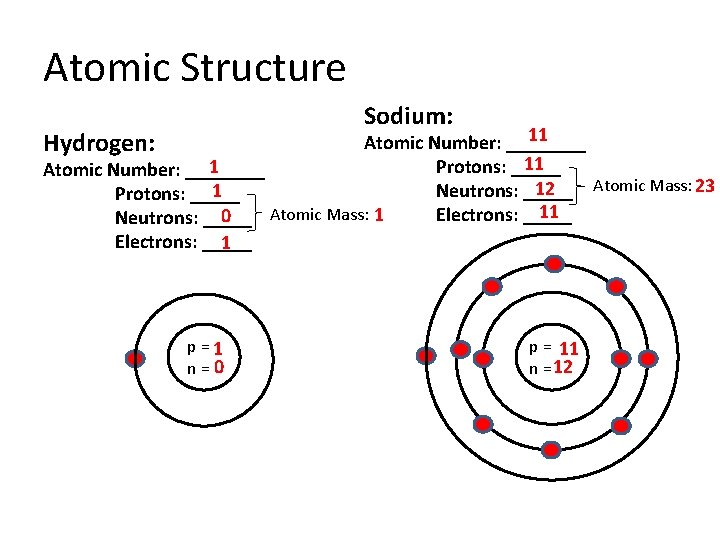
Atomic Structure Sodium: 11 Atomic Number: ____ 11 Protons: _____ 1 Atomic Number: ____ Atomic Mass: 23 12 Neutrons: _____ 1 Protons: _____ 11 Atomic Mass: 1 Electrons: _____ 0 Neutrons: _____ Electrons: _____ 1 Hydrogen: p=1 n=0 p = 11 n = 12