Particle Theory Year 8 Science The particle model

- Slides: 8

Particle Theory Year 8 Science

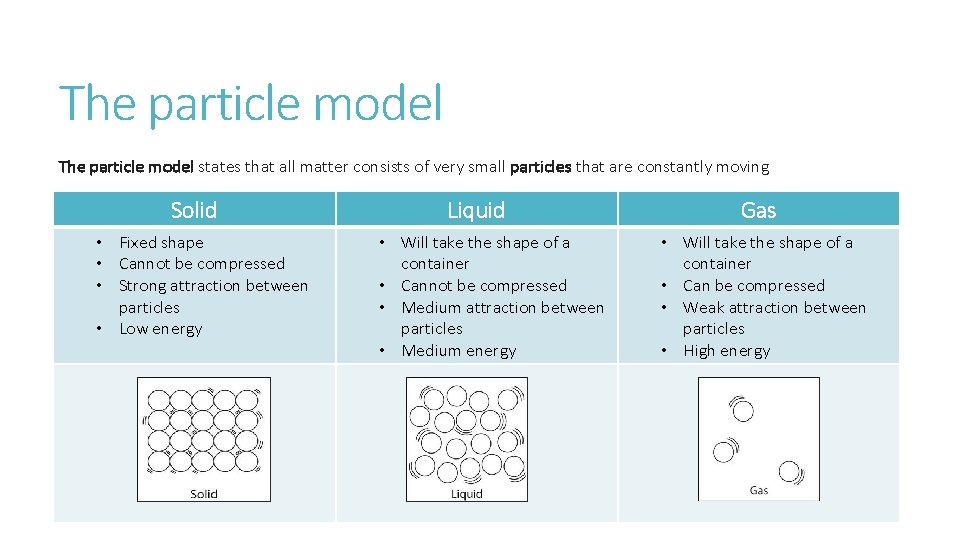
The particle model states that all matter consists of very small particles that are constantly moving Solid • Fixed shape • Cannot be compressed • Strong attraction between particles • Low energy Liquid • Will take the shape of a container • Cannot be compressed • Medium attraction between particles • Medium energy Gas • Will take the shape of a container • Can be compressed • Weak attraction between particles • High energy

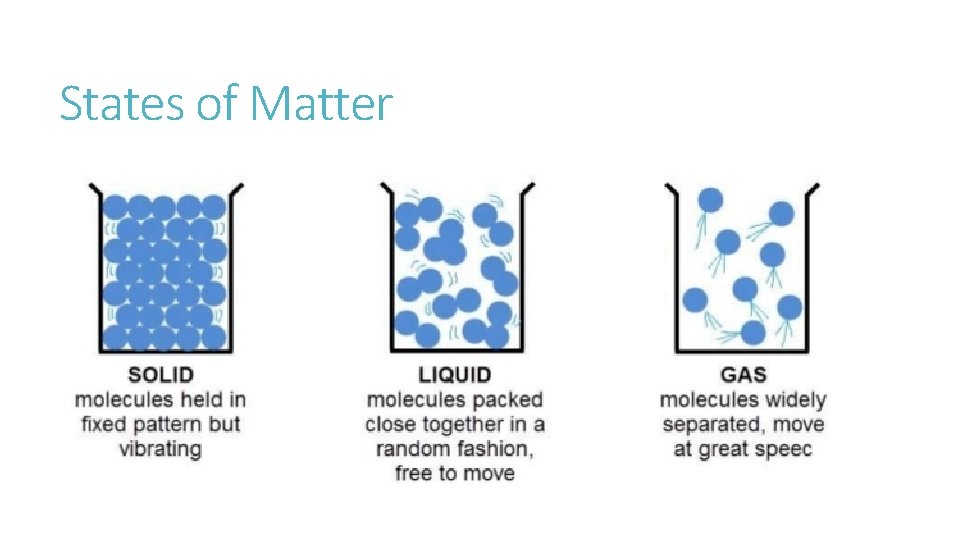
States of Matter

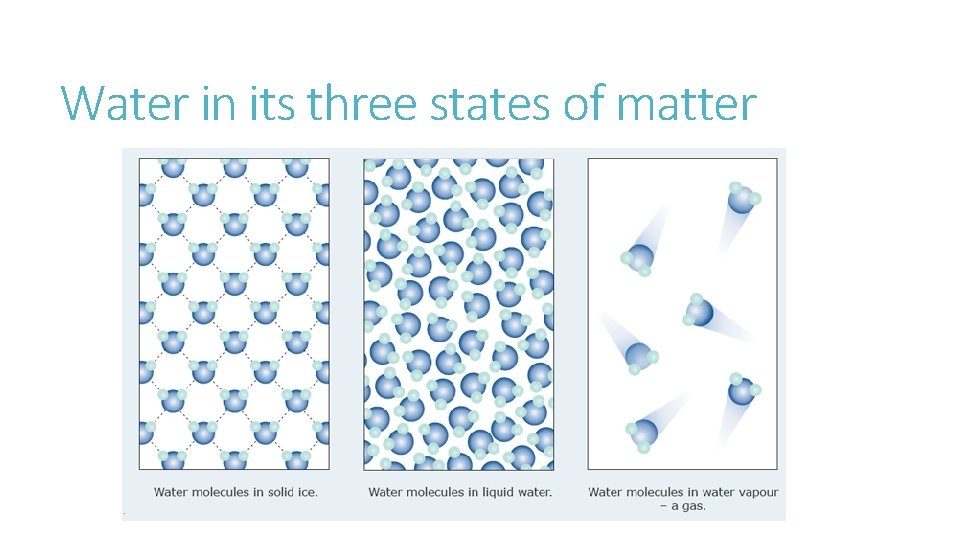
Water in its three states of matter

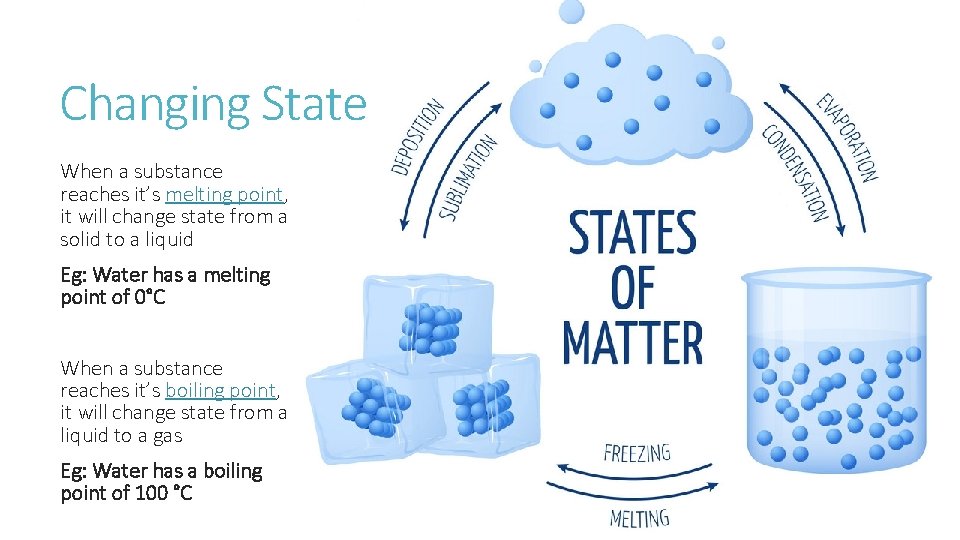
Changing State When a substance reaches it’s melting point, it will change state from a solid to a liquid Eg: Water has a melting point of 0°C When a substance reaches it’s boiling point, it will change state from a liquid to a gas Eg: Water has a boiling point of 100 °C

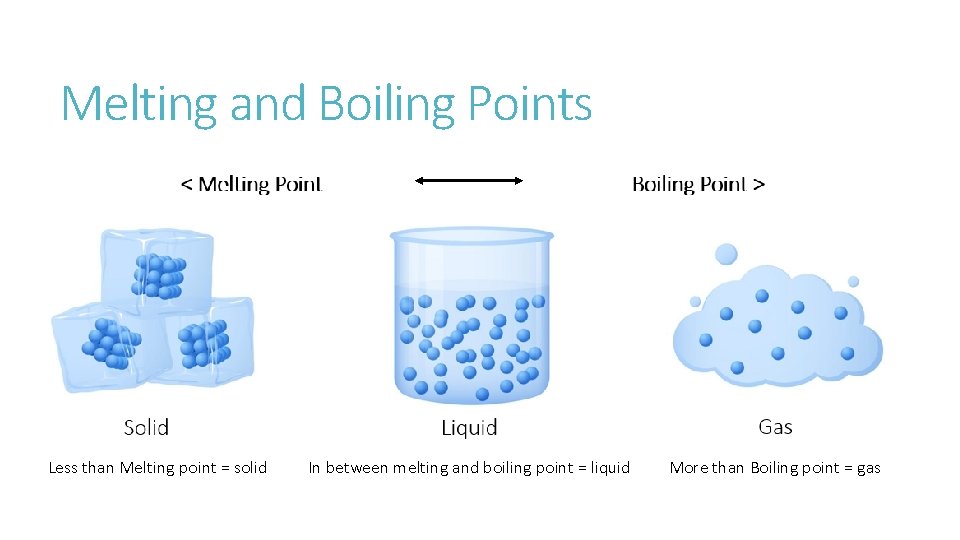
Melting and Boiling Points Less than Melting point = solid In between melting and boiling point = liquid More than Boiling point = gas

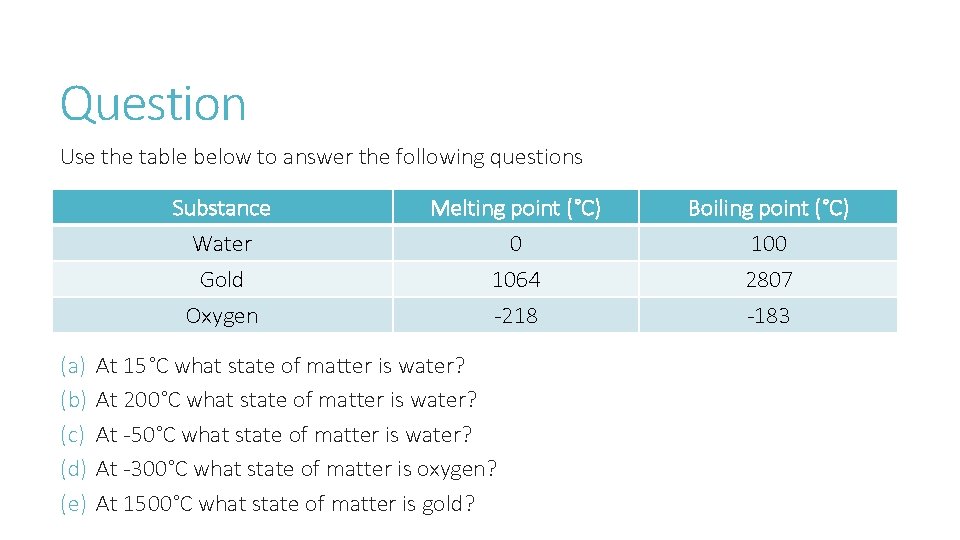
Question Use the table below to answer the following questions Substance Water Gold Oxygen (a) (b) (c) (d) (e) Melting point (°C) 0 1064 -218 At 15°C what state of matter is water? At 200°C what state of matter is water? At -50°C what state of matter is water? At -300°C what state of matter is oxygen? At 1500°C what state of matter is gold? Boiling point (°C) 100 2807 -183

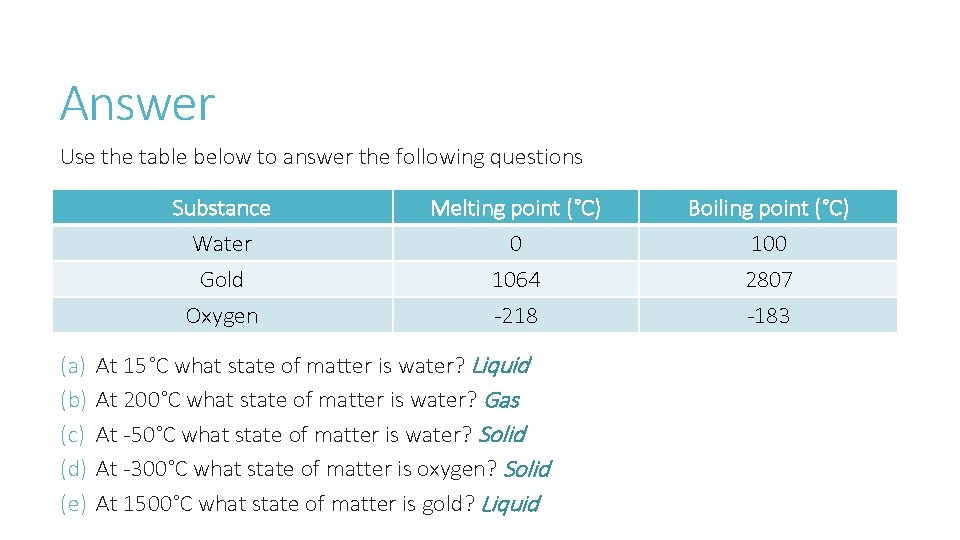
Answer Use the table below to answer the following questions Substance Water Gold Oxygen (a) (b) (c) (d) (e) Melting point (°C) 0 1064 -218 At 15°C what state of matter is water? Liquid At 200°C what state of matter is water? Gas At -50°C what state of matter is water? Solid At -300°C what state of matter is oxygen? Solid At 1500°C what state of matter is gold? Liquid Boiling point (°C) 100 2807 -183