Particle Theory of MatterPhysical Transformationsagain Particle Theory of






- Slides: 14

Particle Theory of Matter/Physical Transformations…again

Particle Theory of Matter Review 1. 2. 3. 4. 5. 6. All matter is made up of particles Particles are always moving The spaces between particles are larger than the particles themselves. All particles of the same substance are made of the same particles regardless of state (solid, liquid or gas) Particles are attracted to each other. The closer the particles are the stronger the attraction. Particles move faster when heated and slower when cooled down

Heat vs Temperature- Particle Theory o o o Heat= thermal energy TOTAL energy contained by an object Travels in waves Can change the matter it touches Transfer of energy from hotter body to colder body o o o Temperature= speed How fast the particles move and is NOT determined by the amount of substance The AVERAGE energy of its particles

Particle Theory and Heat- Example o o Bowling: you throw the ball towards the pins that splits up the pins as the ball hits Pins go in all different directions (like particles) at different speeds The total movement of all the pins is thermal energy (heat) The harder you throw the ball the higher thermal energy

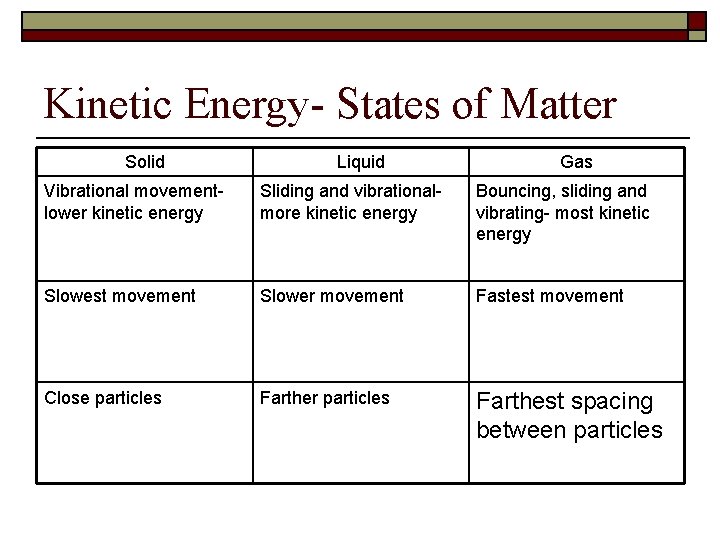
Kinetic Energy- States of Matter Solid Liquid Gas Vibrational movementlower kinetic energy Sliding and vibrationalmore kinetic energy Bouncing, sliding and vibrating- most kinetic energy Slowest movement Slower movement Fastest movement Close particles Farther particles Farthest spacing between particles

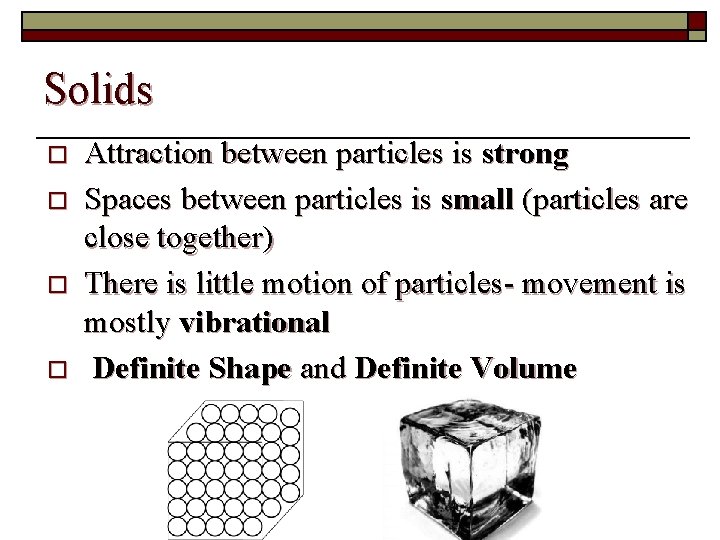
Solids o o Attraction between particles is strong Spaces between particles is small (particles are close together) There is little motion of particles- movement is mostly vibrational Definite Shape and Definite Volume

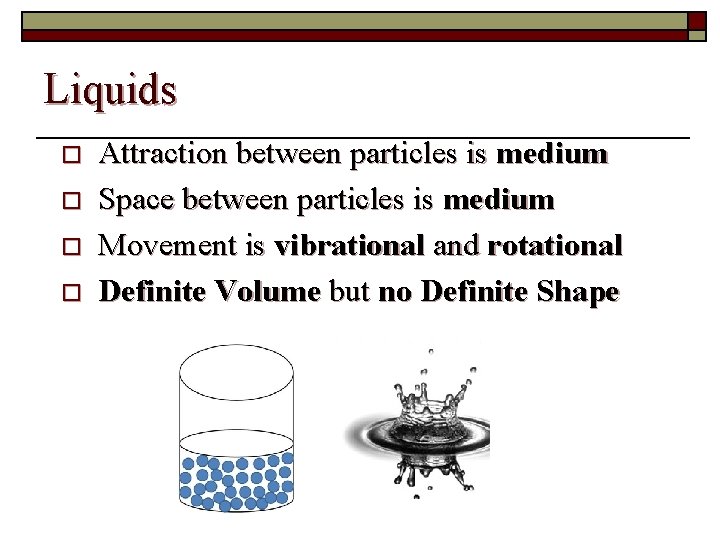
Liquids o o Attraction between particles is medium Space between particles is medium Movement is vibrational and rotational Definite Volume but no Definite Shape

Gases o o Attraction between particles is weak Space between particles is large Movement is vibrational, rotational and translational No Definite Shape and No Definite Volume

Law of Conservation of Energy o o o Energy is NOT a substance Energy is the property or quality of an object that gives it the ability to move, do work Law of Conservation of Energy: Energy CANNOT be created or destroyed. It can only be transformed from one type to another or passed from one object to another

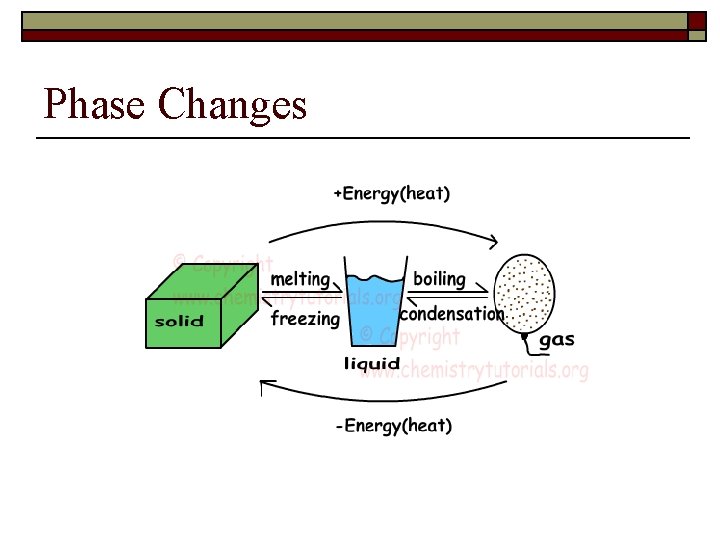
Phase Changes

Phases of Matter- Transformations o o o Energy (heat) causes matter to change state By adding energy you can change solids liquids and liquids gases By removing energy you can change gases liquids and liquids solids

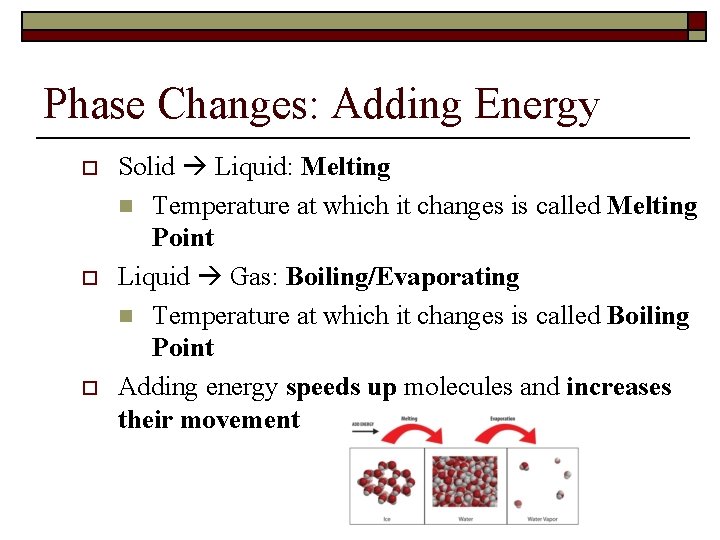
Phase Changes: Adding Energy o o o Solid Liquid: Melting n Temperature at which it changes is called Melting Point Liquid Gas: Boiling/Evaporating n Temperature at which it changes is called Boiling Point Adding energy speeds up molecules and increases their movement

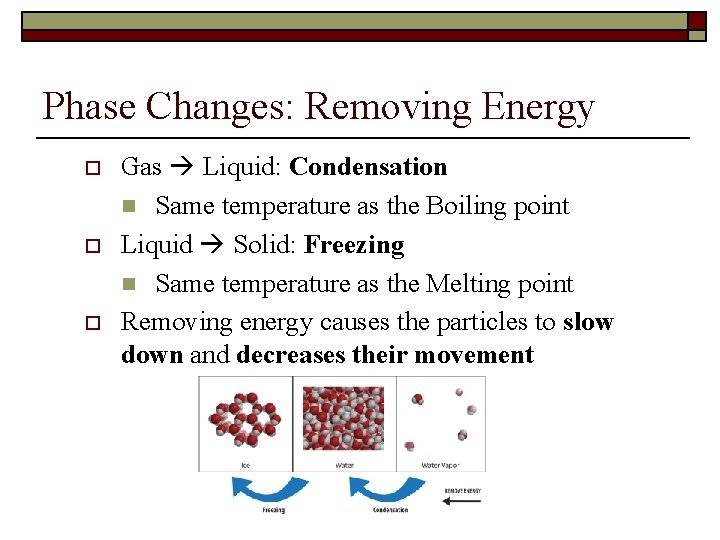
Phase Changes: Removing Energy o o o Gas Liquid: Condensation n Same temperature as the Boiling point Liquid Solid: Freezing n Same temperature as the Melting point Removing energy causes the particles to slow down and decreases their movement

Thermal Pollution