PARTICLE THEORY OF MATTER SCIENCE UNIT 2 WHAT


























- Slides: 33

PARTICLE THEORY OF MATTER SCIENCE UNIT 2

WHAT IS MATTER? • Everything is made of matter • Definition of matter: anything that has weight and takes up space • Weight – how heavy something is • Volume – the amount of space taken up by something (example: water in a glass)

STATES OF MATTER • All matter comes in different forms, or states • 1 – LIQUID • 2 – SOLID • 3 – GAS • All states take up space and have weight

HOW IS AIR MATTER? • If it is, it should take up space and have weight… • Take a few minutes to explain your observations and answer the question above.

CONCLUSION • The balloon full of air will always weigh more than the empty one because: • Air is matter, and matter has weight and takes up space, whether it is a liquid, a solid, or a gas.

WHAT IS MATTER MADE OF? • Matter is made of particles • Particles are: the tiny building blocks that make up matter • Particles cannot be seen with the naked eye

PROPERTIES • Properties are how an object looks and behaves. – Distinguishing qualities such as colour, shape, size, temperature, or weight (characteristics). – Remember the 3 states of matter?

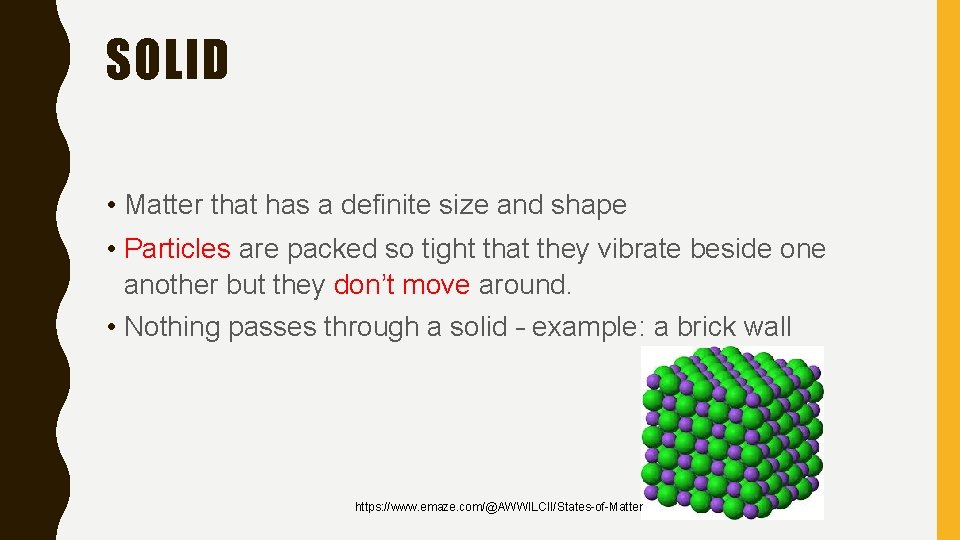
SOLID • Matter that has a definite size and shape • Particles are packed so tight that they vibrate beside one another but they don’t move around. • Nothing passes through a solid – example: a brick wall https: //www. emaze. com/@AWWILCII/States-of-Matter

LIQUID • Matter has definite size but not definite shape. • Particles are packed less tight. The extra room between them allows them to move around. • Example: swimming laps through a pool of water. https: //chemstuff. co. uk/academic-work/year-7/particle-model-of-solids-liquids-andgases/

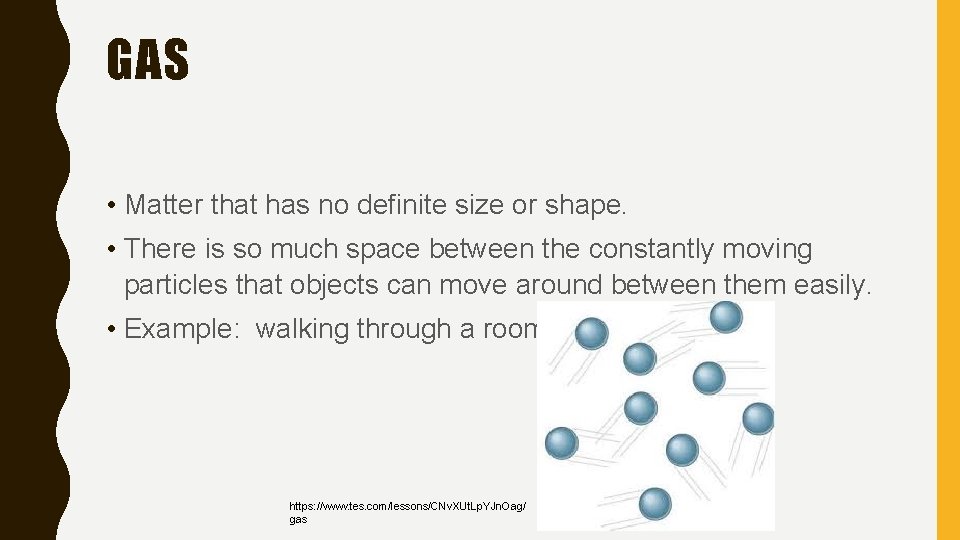
GAS • Matter that has no definite size or shape. • There is so much space between the constantly moving particles that objects can move around between them easily. • Example: walking through a room. https: //www. tes. com/lessons/CNv. XUt. Lp. YJn. Oag/ gas

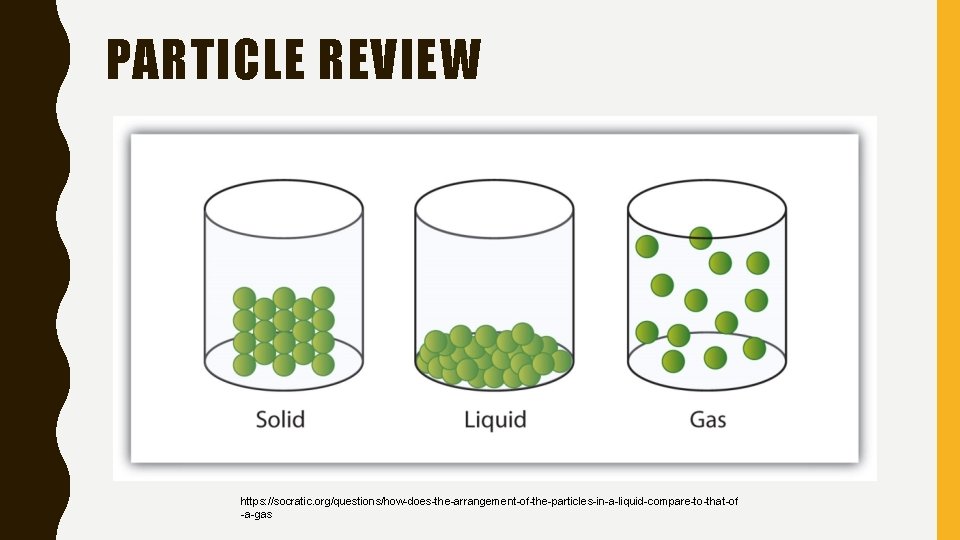
PARTICLE REVIEW https: //socratic. org/questions/how-does-the-arrangement-of-the-particles-in-a-liquid-compare-to-that-of -a-gas

IF SOMETHING IS A SOLID WILL IT STAY A SOLID? • Is a liquid always a liquid? • Do gases ever become non-gases?

THE CANDLE • List the sensory properties of the candle. • Explain your observations. • SOLID LIQUID

MY TEA • List the sensory properties of tea. • Explain your observations. • LIQUID GAS

WINTER TREES • List the properties of water/liquid • Explain the photo • GAS SOLID

CONCLUSION • When an object made of matter changes states, its properties can change. • It doesn’t matter what state an object is in – it’s still matter • Matter is made of tiny particles that are always moving and can change states.

MEASURING PROPERTIES OF MATTER • Hunting for Properties https: //www. youtube. com/watch? v=ZZYn. ERZe 3 Cg (4 min) • Measurement Mystery https: //www. youtube. com/watch? v=7 omxm. CDp. W 7 U (3: 45)

CONCLUSION • Ways to measure properties of matter: • Length (distance from end to end) • Width (distance from side to side) • Height (distance from top to bottom) • Volume (amount of space an object takes up: length x width x height) • Weight (how heavy an object is).

OTHER PROPERTIES OF MATTER: • The science of lunch https: //www. youtube. com/watch? v=d. N 2 VDcmu. Tl 4 • Hardness – the measure of how difficult it is to scratch or crush a certain type of matter • Malleability – the measure of how easily matter can be shaped into different forms (play doh is maleable)

… • Conductivity – measure of how easily matter transports heat or electricity • Magnetism – measure of how matter reacts to a magnet

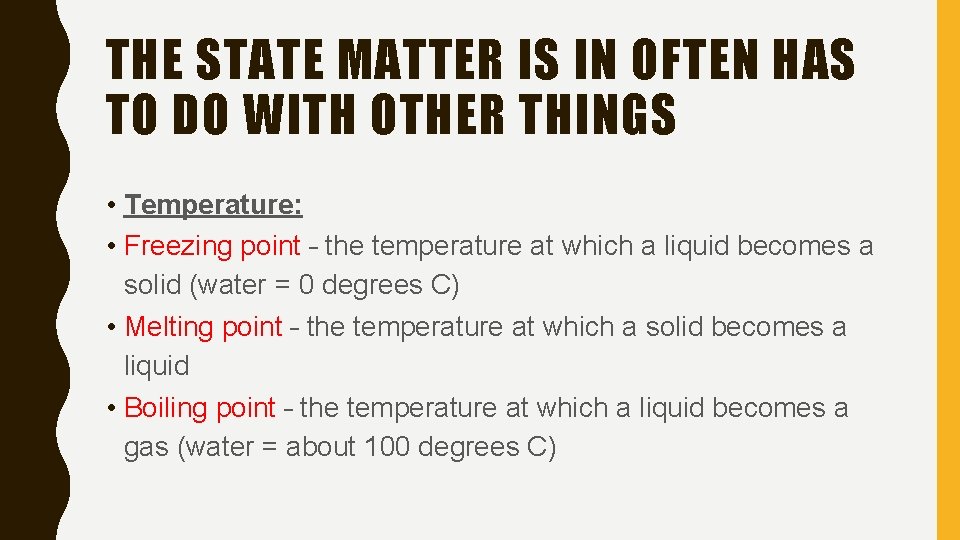
THE STATE MATTER IS IN OFTEN HAS TO DO WITH OTHER THINGS • Temperature: • Freezing point – the temperature at which a liquid becomes a solid (water = 0 degrees C) • Melting point – the temperature at which a solid becomes a liquid • Boiling point – the temperature at which a liquid becomes a gas (water = about 100 degrees C)

… • Reflectivity: • The measure of how much matter reflects light • Metal has high reflectivity • Cloth doesn’t bounce light in the same way • Transparency: • Measure how much light can pass through • Windows have high transparency, bricks have low transparency

MATERIAL SCIENTISTS • Someone who invented a new material to solve a problem. • You are your partner are to research a material that was invented for a purpose. • Answer the 5 Ws and 1 H. • Record your sources using MLA formatting (you should have at least 2) • You will make a 8 x 11 poster of your findings to be put on display in the classroom.

NON-NEWTONIAN FLUIDS • Matter that fits into more than one category (liquid, gas, and solid). • What are some examples? • Reminder: • Solid – has definite size and shape • Liquid – has a definite size but not shape • Gas – has no definite size or shape

EXPERIMENT: • Non-Newtonian Recipe: • 1 ½ c Cornstarch + 1 c Water + bowl + spoon • Put cornstarch in bowl and slowly add water • Stir until it has a texture like honey • Place the material in your hand squeeze to form a solid ball • Now release the pressure

VISCOSITY • The rate at which a fluid flows. • Newtonian fluids (normal ones) flow at a consistent rate. • Non-Newtonian fluids flow at a different rate, depending on how much force or pressure is applied to them. • Our experiment flowed at much slower rate with pressure – acting like a solid • When pressure was removed it flowed faster – acting like a liquid

CONCLUSION • Some materials can fit into more than just one state = non-newtonian fluids

MIXTURES • Any thing made by combining two or more different things

SOLUTIONS • A mixture in which 2 kinds of particles are evenly distributed in a container • Sugar and water • The air we breath (nitrogen, oxygen, other) • Solutions can be separated again, back into the substances that made them

SOLUBILITY • The measure of a substance’s ability to be dissolved • Ex) sand doesn’t dissolve in water = low solubility – The sugar mentioned before has high solubility – But what if I keep adding sugar?

SATURATION • The point at which no more solute can dissolve in a solution – When the sugar sits in the bottom of the water glass…

CHEMISTRY • The science of different kinds of matter, and how that matter can change • Chemical change – mixing things together making a whole new substance (particles are rearranged) – can’t be undone – Gives off or takes heat – Often change colour – Make smells (not always bad) – Release light – Release gases

HEAT • Can be transmitted in 3 ways: conduction, convection, radiation • Bill Nye the Science Guy: Heat https: //www. youtube. com/watch? v=Hnqb. QVPKldk