Particle Theory of Matter Kinetic Theory of Matter


- Slides: 7

Particle Theory of Matter (Kinetic Theory of Matter) ü All matter is made of tiny particles called ATOMS. ü Atoms are always moving (they have kinetic energy). üThe faster the particles move higher the temperature of matter. ü A state of matter is the form in which matter exists: solid, liquid, gas. üThe differences between solids, liquids & gases can be explained with this theory.

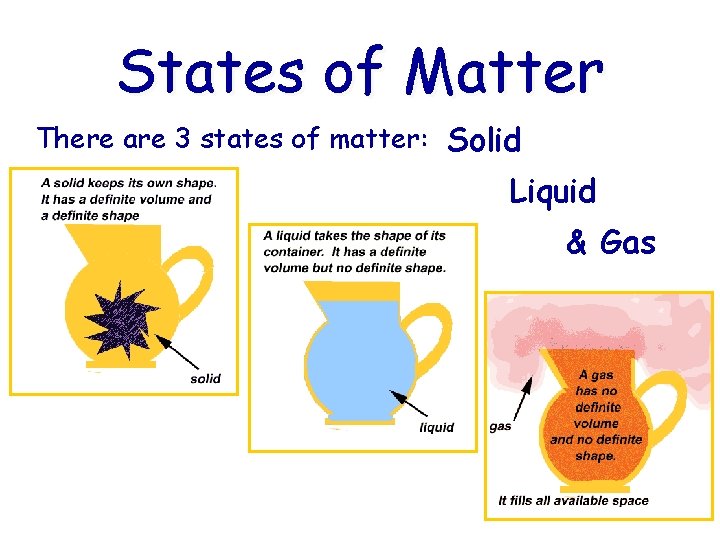
States of Matter There are 3 states of matter: Solid Liquid & Gas

States of Matter

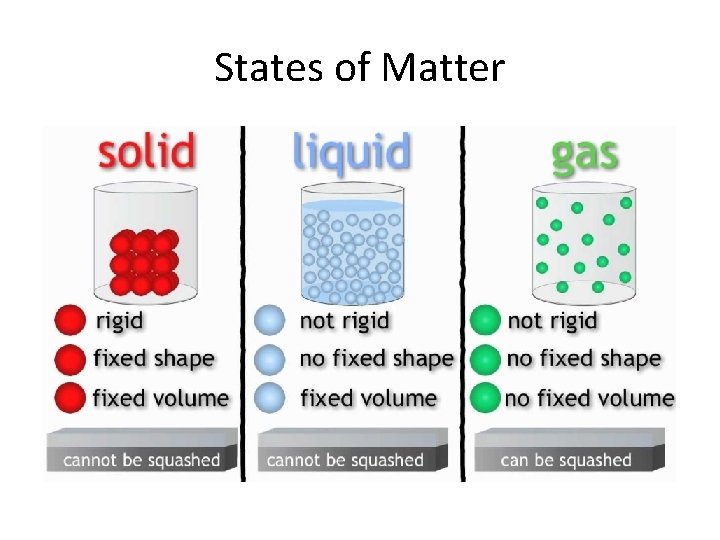
• Have a fixed volume Solids • Can’t be compressed into a smaller volume • Keeps it shape even when moved • Very dense • Have a fixed volume Liquids • Can’t be compressed into a smaller volume • Takes the shape of its container • Flows easily • Dense • Does not have a fixed volume Gases • Can be compressed into a smaller volume • Will fill available space • Flows easily • Not dense

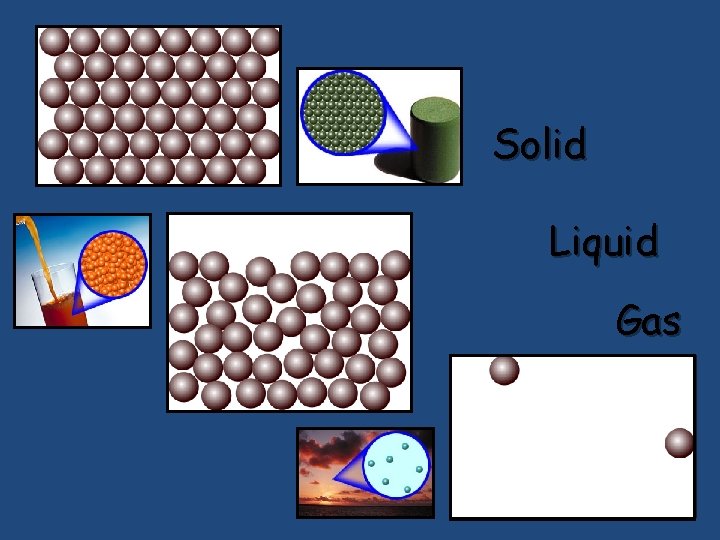
Solid Liquid Gas

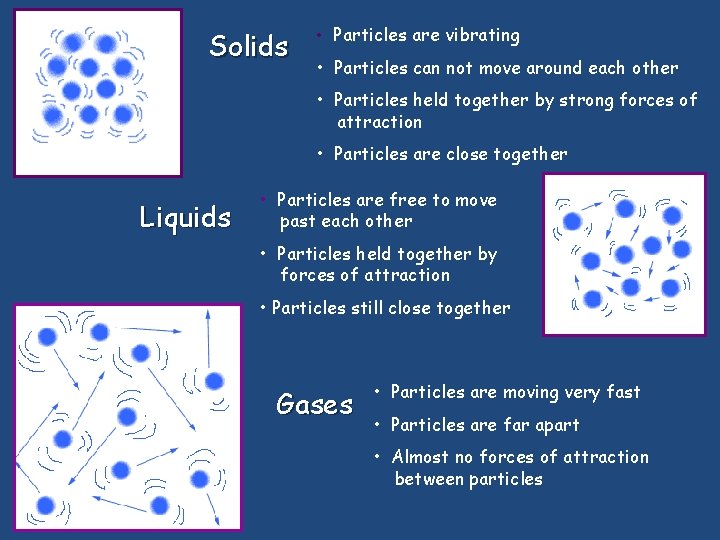
Solids • Particles are vibrating • Particles can not move around each other • Particles held together by strong forces of attraction • Particles are close together Liquids • Particles are free to move past each other • Particles held together by forces of attraction • Particles still close together Gases • Particles are moving very fast • Particles are far apart • Almost no forces of attraction between particles

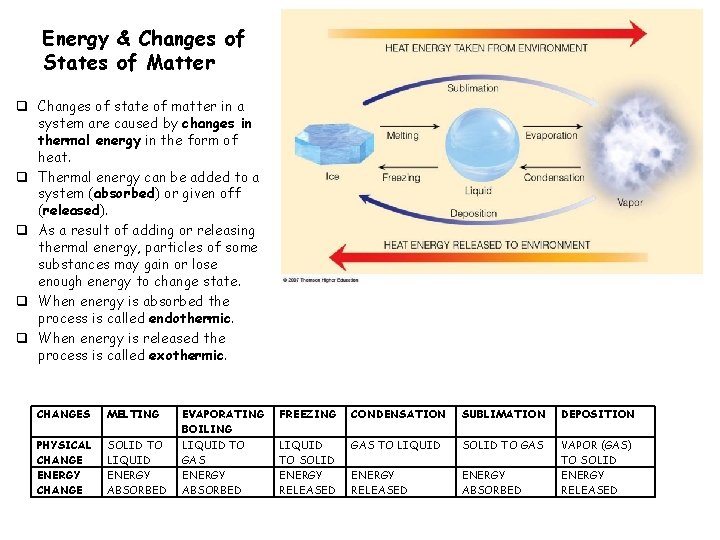
Energy & Changes of States of Matter q Changes of state of matter in a system are caused by changes in thermal energy in the form of heat. q Thermal energy can be added to a system (absorbed) or given off (released). q As a result of adding or releasing thermal energy, particles of some substances may gain or lose enough energy to change state. q When energy is absorbed the process is called endothermic. q When energy is released the process is called exothermic. CHANGES MELTING PHYSICAL CHANGE ENERGY CHANGE SOLID TO LIQUID ENERGY ABSORBED EVAPORATING BOILING LIQUID TO GAS ENERGY ABSORBED FREEZING CONDENSATION SUBLIMATION DEPOSITION LIQUID TO SOLID ENERGY RELEASED GAS TO LIQUID SOLID TO GAS ENERGY RELEASED ENERGY ABSORBED VAPOR (GAS) TO SOLID ENERGY RELEASED