PARTICLE THEORY All matter is made up of







- Slides: 10

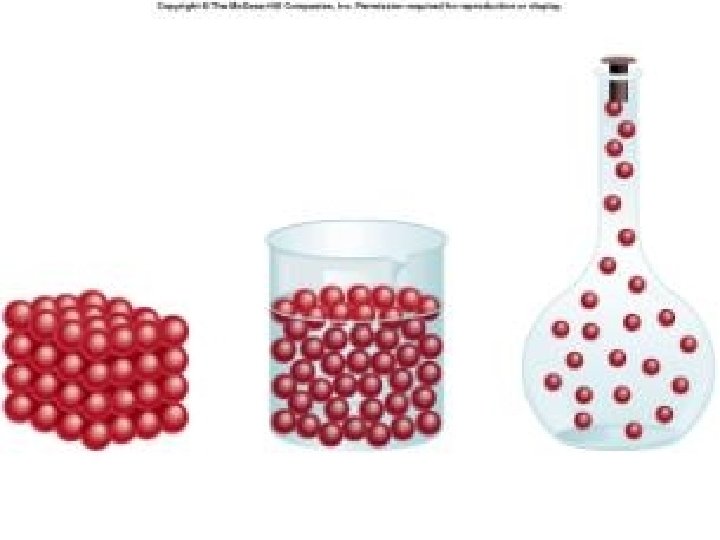
PARTICLE THEORY • All matter is made up of very small particles. • All particles in a pure substance are the same. • There is space between the particles. • The particles are always moving. • The particles in a substance are attracted to each other. The strength of the attraction depends upon the type of particle.


Solid - Particles in a fixed order and location - Particles can only move in their fixed location - Cannot be compressed - Cannot flow

Liquids - Particles not in a fixed order or - Particles move freely, but slowly - Cannot be compressed easily - Can Flow - Takes the shape of the container it is pour into

Gases - Particles not in any organized pattern - Particles move freely and very quickly - Can be compressed - Takes the shape of the entire container

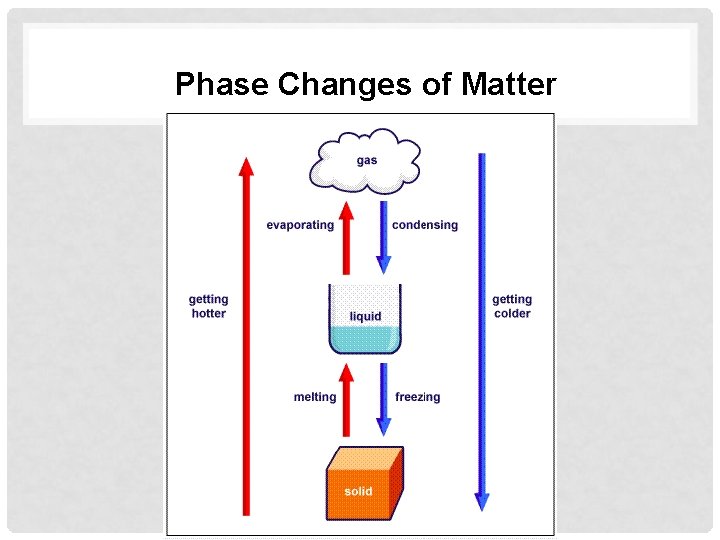
Phase Changes of Matter

Heat and Particle Theory - Heat of fusion – The amount of energy required to turn a sample of solid matter into a liquid (ex. Ice to water) - Heat of Vaporization – The amount of energy required to turn a sample of liquid matter into a gas

Temperature and Theory of Kinetic Energy - Kinetic Energy – Energy produced by the constant motion and collision of particles of matter - - Temperature – The measure of the amount of kinetic energy in matter How are Kinetic Energy and Temperature related?

DENSITY OF SOLIDS, LIQUIDS & GASES • Does water as a liquid, water a solid, or water as a gas have the highest density? Explain.

DENSITY • Density can be described as the “crowdedness” of the particles that make up matter. • When you describe something as being “heavy” or “light” you are referring to that something’s density. • Each substance has its own, unique density. • The closer the particles are together in a substance, the higher its density is.